News From The “TEAM” Study: Risperidone Superior To Valproate And Lithium In Childhood Mania
A symposium at the Annual Meeting of the American Association of Child and Adolescent Psychiatry discussed the Treatment of Early Age Mania (TEAM) study, which comprised 5 different sites in Pittsburgh, Washington DC, Baltimore, St. Louis, and Cleveland. This randomized partially blinded study compared risperidone, valproate, and lithium for the treatment of children with bipolar I mania.
Participants were all severely ill with a Clinical Global Assessment of Severity score (C-GAS) of less than 60 (the mean was 39, indicating that the children were substantially impaired). More than three quarters had psychosis (i.e. hallucinations or delusions) and 99% had dramatic mood shifts within a day (ultradian cycling). All the children had the cardinal symptom of elevated mood.
Among the 290 participants, there was a high incidence of Axis I comorbidities, with 98% of patients having a disruptive behavioral disorder, 77.3% an anxiety disorder, 31% some form of sleep disturbance, and 17% an elimination disorder, of which 15% had enuresis (bedwetting). Nightmares were present in 25.9% of the sample, sleepwalking in 7.2%, and night terrors in 4.8%.
For the purposes of the study, response was considered to have been achieved when a child received a rating of 1 (not ill) or 2 (minimally ill) on the Clinical Global Impressions scale modified for bipolar illness (CGI-BP).
The children (age 6 to 15 with a mean age of 11) were randomized to treatment for 8 weeks with lithium, valproate, or risperidone. Lithium treatment reached blood levels of 1.1 to 1.3mEq/L, valproate reached levels of 111 – 225µg/ML, and risperidone doses were up to 3mg per day. Children who were taking psychomotor stimulants for treatment of ADHD remained on the stimulants after randomization to one of the three drugs. While the treating physicians and clinicians were not blind, blind ratings were performed at week 8.
With a response rate of 68.5%, risperidone was superior to lithium (35.6%) and valproate (24%) based on CGI-BP scores. The mean dose of risperidone was 2.6mg +/- 1.2 per day. The mean blood level at week 8 for lithium was 1.1mEq/L and for valproate was 114µg/ML.
The number of children who improved sufficiently for their C-GAS scores to rise above 60 was also greater for risperidone at 48.3% compared to lithium at 26.7% and valproate at 17.0%.
Editor’s Note: These are among the first controlled data in children with mania below the age of 10 (which is the minimum age for most FDA-approved studies of second-generation antipsychotic drugs). Nonetheless the findings were highly consistent with data in older children and adolescents.
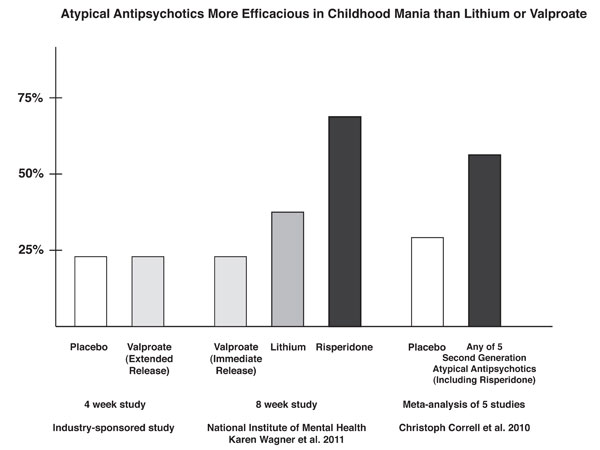
As illustrated in the graph showing results of several similar studies, response to extended release valproate (24%) did not significantly exceed that of placebo (23%) in a randomized industry-sponsored study in older children with mania. These numbers resemble the 24% response rate to immediate-release valproate in the TEAM study of younger children. In a meta-analysis by Correll in 2010 that analyzed data from 5 industry-sponsored studies of second-generation atypical antipsychotics, the rate of placebo response for bipolar mania was 29%, while the response to a second-generation atypical antipsychotic averaged 58% (approaching the 69% response to risperidone observed in the TEAM study).
There were differences in the apparent magnitude of response to both lithium and valproate in this TEAM study compared to open randomized data from 2000, in which Robert Kowatch et al. showed that response to valproate, lithium, and carbamazepine were comparable in children with acute mania. In that study the response to valproate was about 50% and slightly but not significantly higher than the response to lithium and carbamazepine. Response to each of the three drugs was in a range similar to that of risperidone in the current study. Thus these discrepancies remain to be clarified.
The TEAM researchers used statistical analyses to determine which variables might be associated with clinical response. A univariate analysis showed that older age and being African-American predicted a more favorable response, while having comorbid ADHD was associated with worse response. In a multivariate analysis, conduct disorder emerged as a predictor of poor response, and there were significant differences in response of large magnitude across the five different sites used in this study.
The Pittsburgh and Washington, DC sites had much higher responses to lithium and valproate (similar to risperidone) than the other three sites in St. Louis, Cleveland, and Baltimore, which showed the same pattern of response noted in the overall group, i.e. lithium and valproate were inferior to risperidone. The reasons for these differences across sites was not clear, although the sites with better response to lithium and valproate also had the lowest dropout rate.
Interestingly, the dropout rate was higher on lithium and on valproate than on risperidone (32.2% for lithium, 26.0% for valproate, and 15.7% for risperidone). Dropouts for worsening of clinical symptoms were also significantly higher on lithium (13.8%) and valproate (11.5%). There were no drops for worsening of symptomology on risperidone. Dropouts for side effects were not significantly different across the three drugs.
There were differences in side effects. Weight gain was greater on risperidone (averaging 3.3kg compared to 1.42kg on lithium and 1.67kg on valproate). Prolactin increases were substantially greater on risperidone (37.6 units) compared to negligible increases on lithium and valproate. Low-density lipoproteins (LDL cholesterol, the “bad” kind) increased on risperidone, but decreased on valproate. Thyroid stimulating hormone (TSH) increased on lithium, and platelets decreased on valproate. Gastrointestinal distress was more prominent on lithium as was increased urination, dry mouth, and thirst. Difficulty arousing in the morning was reported at baseline in 28.9% of those randomized to valproate, and this increased to 63.9% by the end of 8 weeks of treatment. All three drugs were associated with reductions in suicidal ideation.
Editor’s Note: In summary, risperidone was superior to lithium and valproate in very young children (aged 6 to 15) with BP I mania, but it had more side effects including weight gain and increases in prolactin and LDL cholesterol. Data from this study and on the efficacy of risperidone were published by Barbara Geller et al. in the Archives of General Psychiatry in 2012.