Vagus Nerve Stimulation Improves Depression When Other Treatments Fail
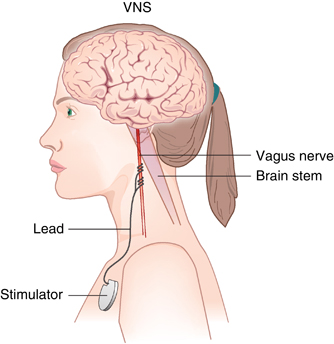 Vagus nerve stimulation (VNS) has been approved by the US Food and Drug Administration as an adjunctive therapy for treatment-resistant unipolar and bipolar depression since 2005. The treatment consists of a pacemaker-like device implanted under the skin in the chest that delivers regular, mild electrical pulses to the brain via the left vagus nerve.
Vagus nerve stimulation (VNS) has been approved by the US Food and Drug Administration as an adjunctive therapy for treatment-resistant unipolar and bipolar depression since 2005. The treatment consists of a pacemaker-like device implanted under the skin in the chest that delivers regular, mild electrical pulses to the brain via the left vagus nerve.
A 2017 study by Scott T. Aaronson and colleagues in the American Journal of Psychiatry reports that over a 5-year period, people with treatment-resistant depression who received VNS did better than those who received treatment as usual. The 795 participants at 61 US sites had either a depressive episode that had lasted for at least two years or had had three or more depressive episodes and had failed to respond to at least four treatments, including electroconvulsive therapy (ECT). Over five years, those who received VNS had higher response rates (67.6% versus 40.9%) and higher remission rates (43.3% versus 25.7%) compared to those who received treatment as usual.
While the study by Aaronson and colleagues was non-blind and non-randomized, it suggests that VNS could be helpful in the long-term management of treatment-resistant unipolar and bipolar depression.
Editor’s Note: VNS was FDA-approved for treatment-resistant seizures in patients aged 12 and older in 1997 and for children 4 years and older in 2017. It was also approved for cluster headaches in 2017. Insurance coverage and reimbursement for VNS is typically available for these neurological conditions, but not for the treatment of depression. This is an unfortunate example of the stigmatization of psychiatric illness—when an FDA-approved device can be kept from people in need of treatment.

