DRAMATIC PROPHYLACTIC RESPONSE TO NIMODIPINE: A Case Report
(This is an invited contribution by Robert Westhead.)
This 50 year old man had a lifetime of incapacitating rapid cycling (10 days up and 10 days down) bipolar I disorder, but then for the past 4 years has had a complete remission on nimodipine (60mg QID). He remains on lithium (800mg), and of his other long-term medications, he has titrated quetiapine down from 800mg to 50mg and has discontinued phenelzine.
He had previously failed to respond to combinations of:
· Lithium
· Anticonvulsant mood stabilizers (including divalproex sodium, lamotrigine, carbamazepine and pregablin)
· Atypical antipsychotics (including quetiapine, aripiprazole and lurasidone)
· Antidepressants (including SSRIs eg citalopram and sertraline, NSRIs eg venlaflaxine and mirtazapine, and a MAOI eg phenelzine)
· Thyroxine
· Propranolol
· Clonazepam
He wanted to highlight this dramatic response to nimodipine in combination with lithium as this dihydropyridine calcium channel blocker is not well known or frequently used for its prophylactic effectiveness.
He noted that as well as stopping the rapid cycling, the nimodipine has provided complete relief from comorbid social anxiety symptoms and remediated cognitive and memory impairment.
This response to nimodipine potentially also has pathophysiological implications. Nimodipine directly blocks the CACNA1C calcium influx gene that has repeatedly been associated with vulnerability to depression, bipolar disorder, and schizophrenia in gene wide association studies. This patient does not know whether he carries this gene variant, but assays for it are routinely available as performed by the company Genomind.
Thus, it remains an open question as to whether those who have the CACNA1C variant would be more responsive to nimodipine compared to those without the variant. Certainly, the efficacy of this agent in treatment of patients with bipolar disorder deserves further consideration and study.
Antihypertensives That Stimulate vs Inhibit Type 2 and 4 Angiotensin II Receptors Decrease Dementia
Marcum et al in JAMA New Open (2023) found that in “57,773 Medicare beneficiaries, initiation of antihypertensive medications that stimulate vs inhibit type 2 and 4 angiotensin II receptors was associated with a statistically significant 16% lower risk of incident dementia, over a median of 6.9 years of follow-up.”
“Angiotensin II receptor type 2 and 4–stimulating antihypertensive medications (hereafter, stimulating medications) included: Angiotensin II receptor type 1 blockers, dihydropyridine calcium channel blockers, and thiazide diuretics.
Angiotensin II receptor type 2 and 4–inhibiting antihypertensive medications (hereafter, inhibiting medications) included: angiotensin-converting enzyme (ACE) inhibitors, ?-blockers, and nondihydropyridine calcium channel blockers.”
Editors Note: If you have hypertension and are at risk for cognitive decline, know that your choice of effective antihypertensive drugs can lead to better cognitive outcomes. Drugs that stimulate the angiotensin II receptor type 2 and 4 help prevent dementia. These drugs include:
ARB type 1, dihydropyridine calcium channel blockers, and thiazide diuretics. (Good guys)
Those that inhibit Angiotensin II receptors types 2 and 4 do not prevent dementia. These drugs include:
ACE inhibitors, beta blockers, and non-dihydropyridine calcium channel blockers. (Bad guys)
Talk with your doc about drugs equally for blood pressure control but those that also have benefits for ultimate preservation of cognition.
Nimodipine Decreases Frontal and Parietal Cortical Activity During Working Memory in Healthy Subjects
At a recent scientific meeting, researcher Kristin Bigos and colleagues described the effects of nimodipine, a treatment for brain hemorrhage, on the brain during working memory tasks. Nimodipine is a dihydropyridine L-type calcium channel blocker. Calcium channel blockers prevent calcium from entering cells in the heart and blood vessel walls, and they are often used to treat high blood pressure.
Nimodipine acts on the CACNA1C calcium influx gene. Certain genetic variations in this gene (particularly the rs1006737 A allele) have been linked to vulnerability to bipolar disorder, schizophrenia, depression, and autism. Carriers of the risk allele also have higher CACNA1C mRNA expression in the dorsolateral prefrontal cortex and exhibit more activity in the frontal and parietal regions of the brain during working memory tasks, suggesting inefficient brain processing in these regions. Bigos and colleagues found that 60mg/day of nimodipine decreased frontal and parietal cortical activity by 39.1% and 42.8%, respectively, during a working memory task, suggesting that nimodipine improved the efficiency of memory processing. Nimodipine’s positive effects were greater in those participants who had the CACNA1C risk allele.
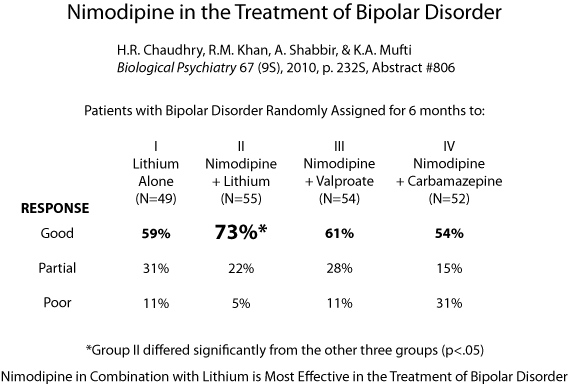
Editor’s Note: Using a placebo-controlled off-on-off-on study design (meaning patients took placebo for a period, then nimodipine, then placebo again and nimodipine again), this editor (Robert M. Post), Peggy J. Pazzaglia and colleagues found that nimodipine had positive effects in both mania and depression in patients with bipolar disorder (described in the 2008 book Treatment of Bipolar Disorder: A Casebook for Clinicians and Patients by Robert M. Post and Gabriele S. Leverich). In a large randomized study of patients with bipolar disorder presented by Haroon R. Chaudhry at the 2010 meeting of the Society of Biological Psychiatry, lithium was associated with about a 50% response rate while the combination of lithium and nimodipine was associated with a 73% response rate.
It remains to be seen whether people with bipolar disorder who have the CACNA1C risk gene would respond better to nimodipine than those without the risk gene, and whether it would improve working memory more in the subgroup with the risk gene.
Calcium Channel May Be Responsible for Circadian Rhythm Abnormalities in Bipolar Disorder
Genetic variation in L-type calcium channel genes have been linked to bipolar disorder. Since calcium plays an important role in circadian rhythms, abnormalities in the calcium channel in bipolar disorder could explain some of the circadian rhythm disturbances patients with bipolar disorder exhibit. New research by Michael McCarthy and colleagues shows that calcium channels in general, and the gene CACNA1C in particular, affect signaling pathways that regulate circadian rhythms in both human and animal cells. The researchers also found that calcium channels affected how lithium changes circadian rhythms, suggesting a mechanism by which the treatment may work. They suggest that drugs that affect the L-type calcium channel may be promising treatments for bipolar disorder.
Editor’s Note: The L-type calcium channel blocker nimodipine has had antidepressant, antimanic, and anticycling effects in some patients with bipolar disorder in small studies both by Peggy Pazzaglia and colleagues (including this author Robert Post) and a larger randomized study by Haroon R. Chaudhry.
The clinical effects of nimodipine results thus align with studies linking the CACNA1C gene to bipolar illness and its early onset, increased expression of the gene in the brain of bipolar patients in autopsy studies, increased levels of calcium in white cells of bipolar patients, and a variety of other neurobiological phenomena observed in normal controls carrying the risk gene.
The new link found between CACNA1C and circadian rhythms further links the L-type calcium channel abnormality and bipolar disorder, as well as the therapeutic effects of the L-type calcium channel blocker nimodipine. This drug deserves further study, especially in those with the genetic variation in CACNA1C that has been linked to bipolar disorder.
A Common Variant of BDNF Predicts Non-Response to IV Ketamine
Brain-derived neurotrophic factor (BDNF) is a protein in the brain that protects neurons and is necessary for long-term memory and learning. Different people have different genetic variations in BDNF depending on which amino acid the gene that codes for it inserts into the protein, valine or methionine. There are three possible combinations that vary in their efficiency. The Val66Val allele of BDNF is the most efficient for secreting and transporting BDNF within the cell body to synapses on dendrites, and is also a risk factor for early onset of bipolar disorder and rapid cycling. Twenty-five percent of the population has a Met variant (either Val66Met or Met66Met), which functions less efficiently. These people have mild decrements in some cognitive processing.
Increases in BDNF are necessary to the antidepressant effects of intravenous ketamine. In animals, ketamine also rapidly changes returns dendritic spines that had atrophied back to their healthy mushroom shape in association with its antidepressant effects. According to research published by Gonzalo Laje and colleagues in the journal Biological Psychiatry in 2012, depressed patients with the better functioning Val66Val allele of BDNF respond best to ketamine, while those with the intermediate functioning Val66Met allele respond less well.
Researcher Ronald S. Duman of Yale University recently found that increases in BDNF in the medial prefrontal cortex are necessary to the antidepressant effects of ketamine. If antibodies to BDNF (which block its effects) are administered to the prefrontal cortex, antidepressant response to ketamine is not observed.
Duman also found that calcium influx through voltage sensitive L-type calcium channels is necessary for ketamine’s antidepressant effects. A genetic variation in CACNA1C, a gene that codes for a subunit of the dihydropiridine L-type calcium channel, is a well-replicated risk factor for bipolar disorder. One might predict that those patients with the CACNA1C risk allele, which allows more calcium influx into cells, would respond well to ketamine.
Gene for Calcium Channel Linked to Bipolar Disorder in Several Ways
No one gene explains the risk of developing bipolar disorder. Many genes are involved, each with a small effect. However, the effects of one particular gene have been validated in multiple different ways. The gene is called CACNA1C, and it codes for one subunit of the dihydropyridine L-type calcium channel. Calcium channels are structures on the membranes of neurons that allow calcium to enter cells and alter their excitability.
Different people can have different variants of the CACNA1C gene, depending on which nucleotides appear there: valine (Val) or methionine (Met). One particular variant (known as the Met/Met single nucleotide polymorphism, rs1006737) has been associated with executive function deficits compared to the Val/Val variant in multiple tests in patients with bipolar disorder. Executive function refers to abilities like planning, organizing, and retaining information. This was reported by Soeiro-de-Souza et al. in the journal Acta Psychiatrica Scandinavica in 2013.
Importantly, CACNA1C has also been linked to risk of bipolar disorder, a finding that was replicated in several large genome-wide association studies (GWAS). Autopsy studies of people who had been diagnosed with bipolar disorder show more calcium channels in their brains. The Met/Met variant of the CACNA1C gene also lets more calcium ions into cells. Those who have the gene variant also show differences in some brain structures known to be involved in the modulation of emotions compared to those without the variant.
In addition to these findings, more than a dozen studies report increased intracellular calcium in the white blood cells of people with bipolar disorder compared to controls. To the extent that these increases in intracellular calcium reflect changes in neurons, this would be consistent with the findings about CACNA1C. High levels of calcium influx and the associated intracellular calcium may increase cellular excitability and potentially dysregulate normal neuronal functioning.
The final piece of evidence linking altered calcium channel regulation to bipolar disorder is a direct therapeutic test of a drug that blocks calcium influx through the dihydropyridine L-type calcium channel. There is evidence that nimodipine, which selectively blocks dihydropyridine L-type calcium channels, has therapeutic effects in bipolar disorder.
Genetic Risk Factors for Onset of Bipolar Disorder
A Genetic Risk Factor For Bipolar Disorder: The CACNA1C Gene
In an abstract presented at the 5th Biennial Conference of the International Society for Bipolar Disorders, Sophia Frangou reported on the CACNA1C polymorphism, a genetic variation that has been associated with the risk of developing bipolar disorder in several genome-wide association studies that search for links between genes and illnesses. Frangou found that those people with the genetic variation had increased volume in some parts of the brain, including the right hypothalamus and the right amygdala, and decreased volume in others, including the putamen, as well as alterations in the functional connectivity of different cortical areas.
These data may be related to findings that calcium influx may play a role in bipolar disorder. In people with the genetic variation, the risk allele binds to a subunit of the voltage-dependent calcium channel, which modulates the influx of calcium from the outside to the inside the neuron.
Increased amounts of calcium are consistently found in the white cells and platelets of patients with bipolar disorder compared to controls. Moreover, the drug nimodipine, a dihydropyridine L-type calcium channel blocker, is effective in the prevention of manic and depressive episodes in a subgroup of patients, particularly those with cycling patterns that are ultra-rapid (4+ episodes per month) or ultradian (including a mood switch within a 24-hour period 4+ times per month). A large randomized study of patients with bipolar disorder presented by H.R. Chaudhry at the 2010 meeting of the Society of Biological Psychiatry also found that while lithium was associated with a 50% response rate, the combination of lithium and nimodipine was associated with a 73% response rate, again suggesting the additional efficacy of blocking L-type calcium channels.
Immune Abnormalities May Predict Onset of Bipolar Disorder in Children at High Risk
At the 5th Biennial Conference of the International Society for Bipolar Disorders E. Mesman discussed connections between immunity and bipolar disorder. Mesman and colleagues followed offspring of parents with confirmed bipolar disorder for 12 years and compared them to children in the general population. In the children of bipolar parents they found higher levels of immune markers called cytokines (PTX3 and sCD25) in circulating monocytes, a type of white blood cell. In the children of bipolar parents they also found a high inflammatory setpoint in the monocytes. T-effector and T-regulatory cells were also different in the offspring of bipolar parents.
While these findings were present in children who had already become ill with bipolar disorder, they were also present in those who had yet to experience a mood disorder, suggesting that these immune and inflammatory markers may ultimately be an important risk marker for the onset of bipolar disorder.
Editor’s Note: These are among the first studies suggesting that immune and inflammatory abnormalities may precede the onset of bipolar disorder. Many studies have shown that patients with active bipolar disorder show more inflammation, including increases in inflammatory markers interleukin 1 (IL-1), interleukin 6 (IL-6), C reactive protein (CRP), and tumor necrosis factor alpha (TNFa). The new data are of considerable importance not only because inflammation could serve as a marker of illness onset, but also because inflammation could become a potential target for therapeutics (i.e. using anti-inflammatory and immune-suppressing agents to treat bipolar disorder).
The Role of Calcium in Genetic Vulnerability, Pathophysiology, and Treatment Of Bipolar Illness
One of the most consistent findings in biological psychiatry is that levels of intracellular calcium in blood elements (platelets and white cells) are higher than normal in patients with mood disorders, particularly bipolar disorder. These data are now supported by genome-wide association studies that have identified a relationship between alterations in a calcium channel and vulnerability to bipolar illness. The specific alteration is in the alpha-IC subunit of the L-type calcium channels, otherwise referred to as CACNA1C. These findings were initially reported by one group funded by the Welcome Trust, a charitable organization that funds health research, in a series of studies that included thousands of patients and controls. Investigator Pamela Sklar later replicated these findings in another large independent sample.
At the 65th Annual Scientific Convention of the Society of Biological Psychiatry, investigator Tyson Tragon reported that there were higher levels of CACNA1C in the cingulate cortex in autopsy specimens of those with bipolar illness than in controls. In a study of mice, some of which had the gene for the glutamate receptor subunit GLuR6 knocked out (i.e. production of the gene was artificially limited), the researchers found that the L-type dihydropyridine calcium channel blocker nimodipine decreased hyperactivity, amphetamine super-sensitivity, risk-taking behavior, and aggression in those with the gene removed. The dihydropyridine-type drugs like nimodipine also decreased stress-related immobilization in the wild type (the animal with normal genes) but not the knockout animals (the ones lacking GLuR6). These data suggest that alterations in a subunit of the dihydropyridine-responsive L-type calcium channel are a risk factor for bipolar illness, a brain abnormality in those who have the illness, and relevant to behavioral/pharmacological models.
Several research groups have noted that treatment with the L-type calcium channel blocker nimodipine (Nimotop) can sometimes have positive effects in mania and depression in those poorly responsive to lithium carbonate. This has been documented by Pazzaglia and Post in double blind off-on-off-on clinical trials (i.e. during off trials patients received placebo and during on trials patients received nimodipine, but the raters were unaware which pill the patient had received). In several instances, a positive response continued when the patient was switched from nimodipine to another dihydropyridine, isradapine (DynaCirc), but not when patients were switched to a different L-type calcium channel blocker, the phenylalkylamine verapamil (sold under the names Calan, Covera, Isoptin, and Verelan), which acts at a slightly different site on the channel. Read more