Cannabis May Produce More Brain Changes in Teens with Bipolar Disorder than in Healthy Teens
At the 2019 meeting of the International Society for Bipolar Disorders, researcher Benjamin Goldstein of Sunnybrook Research Institute in Toronto reported that adolescents with bipolar disorder who smoked marijuana had greater deficits in certain brain areas than did adolescents who did not have bipolar disorder. The areas affected included the dorsal lateral and rostral middle frontal cortex, and middle cortex. Goldstein concluded, “Adolescents with [bipolar disorder] may be particularly sensitive to the neurostructural effects of cannabis.”
Marijuana in general causes adverse changes in brain structure and cognition and vulnerability to paranoia and psychosis. Heavy use in adolescence is associated with an increased incidence of the onset of bipolar disorder and schizophrenia. The Goldstein data suggest several possible causal mechanisms. Those with bipolar disorder may already have brain abnormalities that are exacerbated by marijuana use. Alternatively, marijuana and bipolar disorder together may impact brain structure more than either factor alone would.
Alcohol Use Disorders That Begin Before Age 21 May Cause Lasting Changes to Amygdala
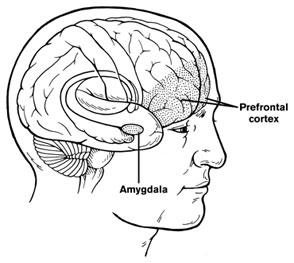 In a 2019 article in the journal Translational Psychiatry, researcher John Peyton Bohnsack and colleagues report that people with alcohol use disorders that began before they were 21 years of age show amygdala changes that people with alcohol use disorders that began after the age of 21 do not appear to have.
In a 2019 article in the journal Translational Psychiatry, researcher John Peyton Bohnsack and colleagues report that people with alcohol use disorders that began before they were 21 years of age show amygdala changes that people with alcohol use disorders that began after the age of 21 do not appear to have.
The amygdalas of those who began abusing alcohol in adolescence showed greater expression of the long non-coding RNA known as BDNF-AS. The increased BDNF-AS was associated with decreased levels of brain-derived neurotrophic factor (BDNF) in the amygdala. BDNF protects neurons and is important for learning and memory.
According to Bohnsack and colleagues, “Adolescence is a critical period in brain development and adolescent drinking decreases orbitofrontal cortex activity and increases amygdala activity leading to less executive control, more emotional impulsivity, alterations in decision-making, and [a higher risk of engaging] in risky behaviors and develop[ing] mental health problems later in life.”
Marijuana Use in Early Adolescence Triples Risk of Psychosis At Age 18
 Hannah J. Jones and colleagues reported in the journal JAMA Psychiatry in 2018 that early- and late-onset marijuana use increased the risk of psychosis at age 18 (odds ratio 3.7 to 2.97). Interestingly, early-onset cigarette use also increased risk of psychosis, but much of the link between cigarette use and psychosis disappeared after correcting for confounding variables.
Hannah J. Jones and colleagues reported in the journal JAMA Psychiatry in 2018 that early- and late-onset marijuana use increased the risk of psychosis at age 18 (odds ratio 3.7 to 2.97). Interestingly, early-onset cigarette use also increased risk of psychosis, but much of the link between cigarette use and psychosis disappeared after correcting for confounding variables.
The data on 5,300 participants born from 1991 to 1992 came from the Avon Longitudinal Study of Parents and Children. Researchers followed up with the participants about their use of marijuana and cigarettes at least three times between the ages of 14 and 19.
Editor’s Note: These data add to a host of epidemiological data that smoking marijuana doubles the risk of psychosis. Risk is further increased among people with a common genetic variant (val/val) of the gene for COMT (catechol-O-methyltransferase), which metabolizes prefrontal dopamine. The variant, which includes two valine amino acids, functions better than other variants that include methionine amino acids. People with val/met or met/met COMT genes metabolize dopamine more slowly, making them relatively protected.
The data are also pretty strong that early heavy use of marijuana is a risk factor for new onset of both bipolar disorder and schizophrenia (and not just an earlier onset in those who might have been vulnerable otherwise).
While marijuana use has become more mainstream with its legalization in many states, its recreational use still carries risks of mental illness. In addition to increasing psychosis risk, marijuana use can also make bipolar disorder more difficult to treat.
A minor component of marijuana, cannabidiol, can have some positive effects, but what you get most of when consuming marijuana is tetrahydrocannabinol (THC), which produces symptoms that resemble psychosis.
Data in rats indicate that a father rat’s use of THC as an adult increases the risk that his offspring (with which he has no contact) will be prone to opiate addiction. The effect is an epigenetic one, conveyed by chemical changes in the father’s DNA that get passed on to the next generation via changes that persist in his sperm. We don’t know if this also happens with humans. So even if you are not worried about your own health, avoiding marijuana use might be good for your children.
Pilot Study Finds Intravenous Ketamine Improves Tough-to-Treat Adolescent Depression
A 2018 open study by Kathryn R. Cullen and colleagues in the Journal of Child and Adolescent Psychopharmacology suggests that intravenous ketamine may improve depression in adolescents who have not responded to at least two antidepressants.
Thirteen patients ranging in age from 12 to 18 with treatment-resistant depression were given six ketamine infusions over a period of two weeks, at doses of 0.5 mg/kg of body weight. A 50% drop in scores on the Children’s Depression Rating Scale-Revised (CDRS-R) was considered a good response, and the average drop in participants’ scores was 42.5%. Five of the thirteen participants (38%) met the criteria for a good response. Three of these participants were still in remission at six weeks, while the other two relapsed within two weeks.
Ketamine was fairly well-tolerated by the young participants. Some had temporary dissociative symptoms or blood pressure changes. Higher absolute doses of ketamine were linked to better response.
The response rates in this group were not as good as in some studies of adults. More research using larger sample sizes and placebo controls is needed to optimize dosing and clarify the safety and efficacy of intravenous ketamine in adolescents with tough-to-treat depression, but this is a promising finding in a small number of adolescents.
Lithium Safely Reduces Mania in Kids 7–17
The first large, randomized, double-blind study of lithium in children and teens has shown that as in adults, the drug can reduce mania with minimal side effects. The study by researcher Robert Findling was published in the journal Pediatrics in October. Lithium is the best available treatment for adults, but until now little research had been done on treatments for children and teens with bipolar disorder.
In the study, 81 participants between the ages of 7 and 17 with a diagnosis of bipolar I disorder and manic or mixed episodes were randomized to receive either lithium or placebo for a period of eight weeks. By the end of the study, those patients taking lithium showed greater reductions in manic symptoms than those taking placebo. Among those taking lithium, 47% scored “much improved” or “very much improved” on a scale of symptom severity, compared to 21% of those taking placebo.
Dosing began at 900mg/day for most participants. (Those weighing less than 65 lbs. were started at 600mg/day.) Dosing could be gradually increased. The mean dose for patients aged 7–11 was 1292mg/day, and for patients aged 12–17 it was 1716mg/day.
Side effects were minimal. There were no significant differences in weight gain between the two groups. Those taking lithium had significantly higher levels of thyrotropin, a peptide that regulates thyroid hormones, than those taking placebo. If thyroid function is affected in people taking lithium, the lithium dosage may be decreased, or patients may be prescribed thyroid hormone.
Lamotrigine Effective in Bipolar I Prevention in Adolescents
At the 2014 meeting of the American Academy of Child and Adolescent Psychiatry, researcher Robert Findling reported on a double blind, placebo controlled 36-week study of lamotrigine for children and adolescents with bipolar I disorder. The doses designed for maintenance treatment averaged about 225 mg/day, achieved by very slow increases over time in order to reduce the risk of a serious rash.
Findling found that lamotrigine was more effective than placebo in extending the time until a patient required an intervention for a new mood episode among the older children in the study (aged 13 to 17). Among the younger children in the study (aged 10 to 12), lamotrigine’s effects were not statistically significant compared to placebo. Findling and colleagues concluded that lamotrigine appeared effective in delaying time to onset of a new episode in adolescents with bipolar I disorder.
Lamotrigine is approved by the Federal Drug Administration (FDA) for bipolar disorder in adults only.





