PREVENT EPISODES, PROTECT YOUR BRAIN, BODY, AND SELF
Kessing and Andersen 2017 wrote:”Overall, increasing number of affective episodes seemsto be associated with:(i) increasing risk of recurrence, (ii) increasing duration of episodes, (iii) increasing symptomatic severity of episodes,(iv) decreasing threshold for developing episodes, and (v) increasing risk of developing dementia.
Conclusion: Although the course of illness is heterogeneous, there is evidence for clinical progression of unipolar and bipolar disorder.”
These adverse outcomes emphasize the importance of early and sustained treatment to prevent the occurrence and accumulation of episodes.
Cariprazine Effective in AD-Resistant MDD
Maletic, V, et al. reported on Efficacy of Adjunctive Cariprazine Across Individual Depressive Symptoms in Major Depressive Disorder: A Post-Hoc Analysis. Poster presented at Psych Congress 2022; September 17-20, 2022.
“With 751 patients (502 on CARIPRAZINE (CAR) and 249 on PBO), the LSMD (95% CI) score change from baseline to week 6 was significant in favor of CAR. Items of the MADRS scale were assessed individually over the course of the study, including apparent sadness, reported sadness, reduced appetite, lassitude, inability to feel, pessimistic thoughts, and suicidal thoughts, showing improvement compared to PBO. “
A NEW VIEW ON MEMORY TO REMEMBER
Steven Ramirez PhD of Boston University gave a talk (8/9/22) on memory for the BBRF hosted by Jeffrey Borenstein President & CEO of the Brain & Behavior Research Foundation. Ramirez showed that positive (food) and negative (shock) memories of different places were stored in different neurons of the hippocampus. If he turned on the positive memories with optical stimulation while a mouse was in a negative memory place and would ordinarily show freezing representing fear behavior, much less freezing occurred with the insertion of the positive memory. Positive memories appear to trump negative memories.
Ramirez found score of genes were activated or turned off in the positive memory cells, some of which, but not all, overlapped with the negative memory cells. Remarkably, the bulk of the unique positive memory genes were related to synaptogenesis and neuroprotection, while the bulk of genes unique to the negative memory cells were related cell death and other toxic factors. Ramirez hopes these data will provide clues to not only helping people with PTSD, but also ultimately providing targets for providing protection against degenerative diseases.
When Ramirez was asked by Borenstein what people could do now, he related his own experience of every morning filling out a form for gratitude and gratefulness for at least 3 things he could be grateful for the previous day or anticipated for the current day. The positive memories that this invoked in him set up his positive and optimistic attitudes for the rest of the day. He recommends this approach of positive memories modulating the current pervasive stressors of the day. For people interested in the details of his experiments summarized above, they should look for the in press articles of Grella et al Nature Communications and Shpokayte et al Nature Communications.
First and Only Oral NDMA Receptor Antagonist for MDD Can Now Be Prescribed
| October 28, 2022 |
| FEATURED NEWS: First and Only Oral NDMA Receptor Antagonist for MDD Can Now Be Prescribed Extended-release dextromethorphan HBr-bupropion HCl (Auvelity™), the only oral N-methyl D-aspartate (NMDA) receptor antagonist approved for the treatment of major depressive disorder (MDD), is now available by prescription in the United States, maker Axsome Therapeutics Inc. announced. The drug is supplied in 105-mg tablets in 30-count bottles.On August 18th, 2022, the US Food and Drug Administration (FDA) approved dextromethorphan HBr-bupropion HCl extended-release tablets 45mg/105mg |
Review Describes Latest Findings on the Mechanisms of Psychedelic Drugs and Their Therapeutic Potential
In a 2021 review article in a special issue of the Journal of Neurochemistry devoted to “Psychedelics and Neurochemistry,” researcher Alaina M. Jaster and colleagues summarized recent findings on psychedelic drugs, including their potential as treatments for psychiatric disorders and the structural changes they produce in the brain. The review article focused on findings in humans and provided background context based on findings in animals, particularly rodents.
In the article, Jaster and colleagues write that psychedelics “have in common a battery of acute (within minutes to hours) effects in humans that include profound changes in processes related to perception, cognition, sensory processing, and mood.” They are not considered to be addictive, and recent research has identified fast-acting and long-lasting therapeutic effects of psychedelics, particularly for the treatment of depression and substance abuse.
While psychedelic drugs interact with the brain in complicated ways, the role of serotonin 5-HT2A receptors seems to be crucial to their effects. Psychedelics have classically been divided into two groups based on their chemical structures: phenethylamines (which include mescaline and its synthetic analog DOI) and tryptamines (which include psilocybin/“magic mushrooms,” DMT/ayahuasca, and ergolines like LSD, which are sometimes separated into a third category). The authors of the review highlight that other substances with different chemical structures that do not fit into this classification can also function as psychedelics. Examples include efavirenz and quipazine, which both activate serotonin 5-HT2A receptors and change rodent behavior in the same way that other psychedelic drugs do. These drugs are providing new directions for research into how psychedelics work at both serotonin 5-HT2A receptors and monoaminergic G protein-coupled receptors (GPCRs).

Rodent studies are often used to investigate how psychedelic drugs work. Rodent behaviors such as ear scratching and head twitching increase when the rodents are given drugs that have psychedelic effects in humans. These behaviors return to normal when rodents are given drugs that function as serotonin 5-HT2A receptor antagonists, preventing the stimulation of these serotonin receptors.
While it has been established that serotonin 5-HT2A receptors play an important role in the hallucinogenic effects of psychedelic drugs, how serotonin receptors are involved in some of the therapeutic effects of psychedelics, such as antidepressant effects and changes to synaptic plasticity, is not yet clear.
According to the review, “A number of studies in animal models as well as postmortem human brain samples from subjects with depression and controls has provided evidence that mood disorders occur in conjunction with a reduction in the density of dendritic spines, particularly in the frontal cortex.” Dendrites are the projections from the cell bodies of neurons upon which nerve endings from the axons of other neurons synapse. The surface of these dendrites is covered in mushroom-shaped spines that help create these synaptic connections. The review describes in vitro and in vivo research on mice that suggests that the psychedelics DOI, DMT, and LSD can remodel dendritic spines.
At a recent scientific meeting, researcher Javier González-Maeso, one of the authors of the review, described findings from a recent study of mice given DOI. The structure of the dendritic spines in the prefrontal cortex changed rapidly in these mice. They had also been conditioned to produce a fear response, and the extinction process to get rid of this learned fear was sped up in the mice given DOI. These effects occurred via the serotonin 5-HT2 receptors. The exposure to the psychedelic also affected the regulation of genes involved in synaptic assembly for days, suggesting that epigenetic-induced changes in synaptic plasticity may underlie some of the long-lasting therapeutic effects of psychedelics.
The review also addressed “microdosing,” the recreational practice of consuming small amounts of psychedelics such as psilocybin or LSD, based on the theory that amounts too small to create a hallucinogenic effect may instead produce therapeutic results. The authors find limited data to support microdosing. Most studies in humans and rodents have found no effect or, in the case of one rat study, a worsening of dendritic spine density following microdosing.
Psilocybin Comparable to Escitalopram in the Treatment of Depression

Four randomized, controlled clinical trials have established that psilocybin, the hallucinogenic compound in “magic mushrooms,” has anti-depressant effects. Recently, a phase 2 clinical trial compared the effects of psilocybin to a US Food and Drug Administration (FDA)–approved treatment for depression, the selective serotonin-reuptake inhibitor (SSRI) escitalopram. The study by Robin Carhart-Harris and colleagues was published in the New England Journal of Medicine in 2021.
The 59 participants in the 6-week study, which took place in the United Kingdom, had longstanding moderate-to-severe major depressive disorder. They were randomized to two groups. The psilocybin group received two separate doses of 25 mg of psilocybin 3 weeks apart, plus 6 weeks of daily placebo. The escitalopram group received two separate doses of 1 mg of psilocybin 3 weeks apart plus 6 weeks of daily oral escitalopram. (The small doses of psilocybin administered to the escitalopram group were assumed to have negligible psychiatric effects.) All participants were told they would receive psilocybin (though not the dose) in order to standardize their expectations.
In addition to the drug treatments, all participants also received psychological support, which consisted of monitoring immediately after the administration of the drugs (given the expectation that the 25mg dose of psilocybin would produce an “altered quality of conscious experience”), psychological debriefings, and an active listening session.
Psilocybin improved depression symptoms to a greater extent than escitalopram did. Among the participants, 70% of the psilocybin group responded to the treatment, compared with 48% of the escitalopram group. Remission occurred in 57% of the psilocybin group compared with 28% of the escitalopram group. These differences in the outcomes for the two groups were not statistically significant.
The FDA has designated psilocybin a “Breakthrough Therapy” for severe depression, which indicates that the therapy may be a substantial improvement over existing therapies for a serious condition. The designation is intended to speed up the drug development and review process.
The state of Oregon legalized psilocybin-assisted therapy in 2020.
MDMA Superior to Placebo in Treatment of Severe PTSD

An article by researcher Jennifer M. Mitchell and colleagues published in the journal Nature in 2021 reported that MDMA was more effective than placebo at treating severe post-traumatic stress disorder in an 18-week phase 3 clinical trial.
The article reported on a randomized, double-blind, placebo-controlled, multi-site trial that took place in the US, Canada, and Israel. Participants in the trial had severe PTSD, and in some cases also had dissociation, depression, a history of alcohol or substance use disorders, or childhood trauma. In the trial, 91 participants were required to stop any current psychiatric medications for a “washout” period, and then were randomized to either a group that received 12 therapy sessions plus placebo or a group that received 12 therapy sessions and 3 doses of MDMA, which were administered in a clinical setting.
Two months after the last therapy session, participants who had received MDMA during the trial showed greater reductions in PTSD symptoms and less functional impairment than those who were assigned to the placebo group during the trial. At the endpoint of the study, 67% of participants in the MDMA group no longer met the criteria for a PTSD diagnosis, compared to 32% of the placebo group.
Side effects that were more prevalent among the MDMA group were typically transient and mild to moderate in severity. These included muscle tightness, decreased appetite, nausea, sweating, feeling cold, and increased blood pressure and heartrate. The MDMA group did not show any increases in drug abuse or suicidality compared with the placebo group, and MDMA had similar effects among those participants with and without comorbid conditions. The authors concluded that MDMA was highly efficacious, safe, and well-tolerated as a treatment for PTSD and urged that further study of the treatment be done without delay.
Currently, US Food and Drug Administration (FDA)–approved treatments for PTSD include the selective serotonin reuptake inhibitors (SSRIs) sertraline and paroxetine, but approximately half of PTSD patients do not respond to these drugs or to evidence-based trauma-specific psychotherapy or cognitive-behavioral therapies. The FDA has designated MDMA-assisted therapy for PTSD a “Breakthrough Therapy.” This designation indicates that the therapy may be a substantial improvement over existing therapies for a serious condition, and is intended to speed up the drug development and review process.
MDMA (or 3,4-methylenedioxymethamphetamine) is the same chemical used recreationally under the name ecstasy or molly. However, the positive effects in the trial followed careful dosing (which is impossible with street drugs) and targeted therapy sessions.
We’re back!
After a pandemic-related hiatus, we’re back, and we hope to bring you articles regularly. For print subscribers, we hope to send out a single large issue in the coming months.
Transcranial Direct Current Stimulation (tDCS) Induces Gray-Matter Increases in Depression
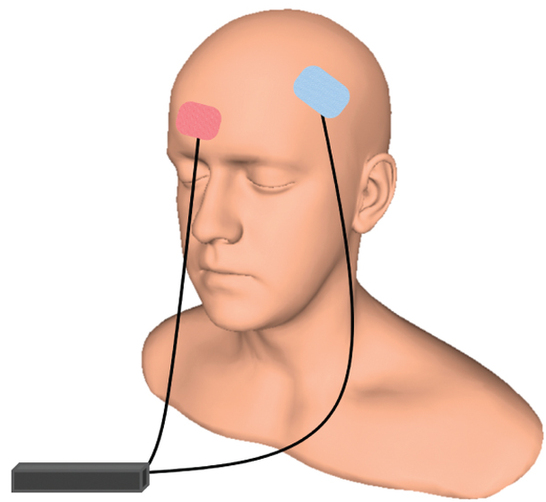
At the 2021 meeting of the Society of Biological Psychiatry (SOBP), researcher Mayank Jog and colleagues described a study of transcranial direct current stimulation (tDCS) in 59 patients with moderate depression. The patients received either tDCS sessions that delivered electrical current at 2mA for 20 minutes or a same-length sham stimulation delivered using a double-blind stimulator, for a total of 12 sessions over 12 consecutive working days. Jog and colleagues found that compared to the sham stimulation, tDCS induced increases in gray matter volume in the left dorsolateral prefrontal cortex (DLPFC) target area, with a statistically large effect size (Cohen’s d = 1.3). The researchers plan to follow up this study that found structural changes to the brain after tDCS with more research on the antidepressant effects of the treatment.
Good Outcomes Among Patients with Major Depressive Disorder Treated with Transcranial Magnetic Stimulation in a Large Study
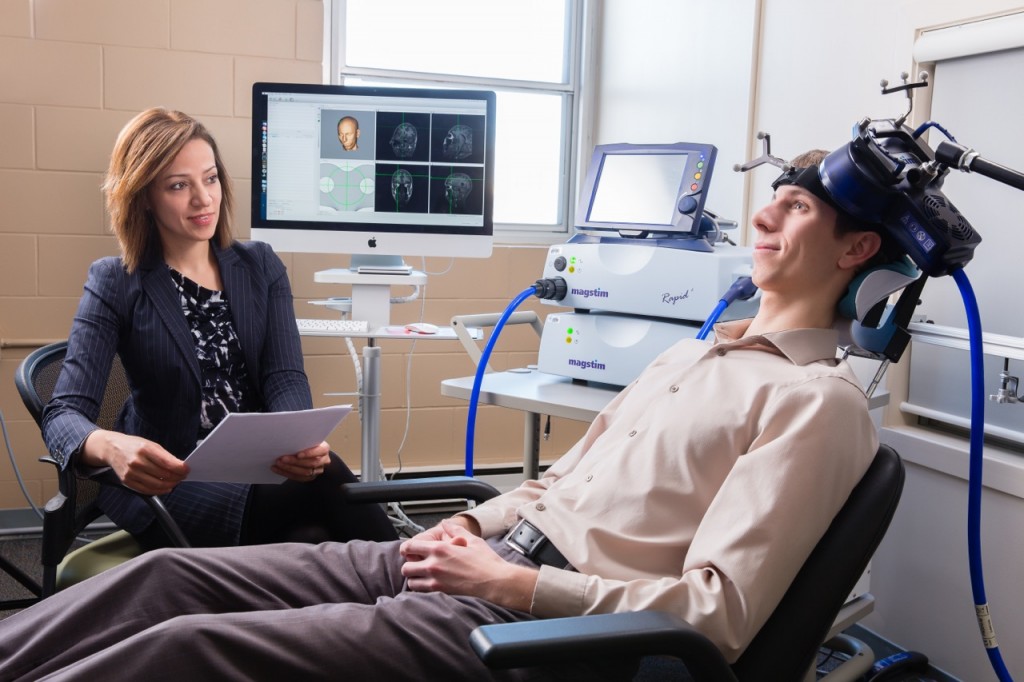
At the 2021 meeting of the Society of Biological Psychiatry (SOBP), researcher Harold Sackeim and colleagues reported on data collected from patients in clinical treatment for major depression who received transcranial magnetic stimulation (TMS) at 103 practice sites. A total of 5,010 depressed patients were included in the intent-to-treat sample, and 3,814 completed the study, meaning that they either reached remission or were treated at least 20 times and went through a final assessment. “Response (58–83%) and remission (28–62%) rates were notably high across self-report and clinician-administered assessments,” and women had better outcomes than men. Sackeim and colleagues concluded, “Strong efficacy and the low side effect and medical risk profile suggest that TMS be evaluated as a first-line treatment for [major depressive disorder].”

