Inflammation Predicts Poor Response to Fluoxetine in Kids
Inflammation upsets the balance of neurotransmitters in the brain and can make antidepressants less effective. In new research by Maya Amitai and colleagues, children and adolescents were less likely to respond to the selective serotonin reuptake inhibitor (SSRI) antidepressant fluoxetine if they had high levels of inflammation measured in the blood.
Amitai’s study included 41 patients between the ages of 9 and 18. They met criteria for a diagnosis of either major depression or an anxiety disorder. The participants were treated with the SSRI fluoxetine for eight weeks. Those with high levels of the inflammatory markers tumor necrosis factor (TNF) alpha, interleukin-6, and interleukin 1 beta were less likely to respond to the antidepressant treatment. The research was published in the Journal of Child and Adolescent Psychopharmacology in 2016.
Editor’s Note: These findings parallel those from studies of adults, suggesting that inflammation can predict poor response to antidepressants in all age groups.
Supplement Acetyl-L-Carnitine May Treat Stress and Depression
N-acetylcysteine (NAC), an antioxidant sold in health food stores, has several beneficial effects on brain and behavior. It improves depression and can reduce cravings for cocaine, alcohol, marijuana, and nicotine, and can also help control habit-driven behaviors such as gambling, compulsive hair-pulling, and symptoms of obsessive-compulsive disorder (OCD).
New research, particularly by researcher Nascaa and colleagues in 2014 and 2016, has identified a related compound, acetyl-l-carnitine (ALC), as an anti-stressor and antidepressant in animals, and researchers have begun to explore its use in people. ALC has been found to improve mitochondrial function and improve recovery from peripheral nerve damage. ALC also inhibits the release of glutamate, which can prevent depressive behaviors following stress.
A 2004 study by P. Ruggenenti and colleagues in the journal Hypertension found that in people, 1 gm of ALC taken twice daily safely improved arterial hypertension, insulin resistance, impaired glucose tolerance, and low levels of adiponectin in the blood (a risk factor for diabetes) in subjects at increased cardiovascular risk.
In a 2014 article in the Journal of Psychiatric Research, researcher S.M. Wang and colleagues reviewed evidence that ALC improves mild depression. Two randomized clinical trials indicated that ALC was more effective than placebo for mild depression. Two other randomized clinical trials showed that ALC was as effective as the antidepressants fluoxetine and amisulpride for mild depression. The supplement was as tolerable as placebo and better tolerated than fluoxetine and amisulpride. Wang and colleagues suggested that more clinical trials are needed to confirm that ALC is effective in depression.
Editor’s Note: If further clinical trials confirm the antidepressant effects of ALC, it could represent a new way to treat chronic stress and depression and regulate insulin. Together these effects could reduce the cardiovascular risks that accompany depression.
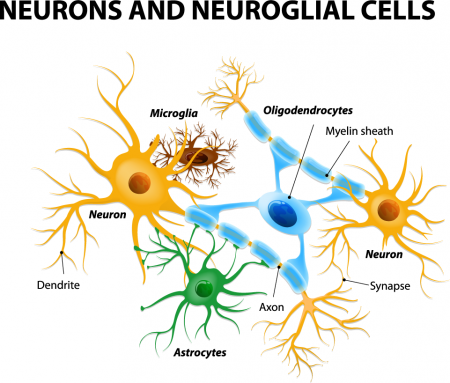
Astrocytes Can Turn Toxic
Astrocytes usually play a helpful role in the brain. These glial cells facilitate neural connections and prune unnecessary ones. However, new research details how infection or trauma can render astrocytes toxic, leading to brain disorders. In a 2017 article in the journal Nature, researcher Shane A. Liddelow and colleagues describe how resting astrocytes can become harmful reactive astrocytes.
The researchers determined that reactive astrocytes are found in brain tissues following brain injuries, or in neurological disorders including Alzheimer’s, Parkinson’s, Huntington’s, amyotrophic lateral sclerosis (ALS), and multiple sclerosis. Liddelow and colleagues also determined that microglia play a role in transforming resting astrocytes into a subtype of harmful reactive astrocytes, dubbed A1 astrocytes, and that the latter secrete a neuron-killing toxin.
A1 astrocytes lose their ability to promote neuronal survival and to create new synapses. The researchers showed that blocking the formation of A1 astrocytes prevented certain neuron deaths, and they hope this finding will lead to new treatments for brain injuries and neurological disorders.
Editor’s Note: It is possible that A1 astrocytes are also induced in patients with bipolar disorder, post-traumatic stress disorder (PTSD), and schizophrenia, as well as head traumas and neurological disorders.
Mysteries Remain in the Relationship Between Inflammation and Depression
At the 2017 meeting of the American College of Psychiatrists, researchers Charles L. Raison and Vladimir Maletic gave a plenary lecture on the role of inflammation in depression. Meta-analyses have confirmed that inflammatory markers including Il-1, Il-6, TNF alpha, and CRP are elevated in about 1/3 of depressed patients. However, Raison and Maletic made the point that anti-inflammatory medications are not for everyone. While patients with elevated levels of CRP at baseline responded to an anti–TNF alpha antibody, patients with low CRP values at baseline actually got worse.
Raison and Maletic cited three studies that have also linked CRP to differential response to traditional antidepressants. In unipolar depression, those with low CRP respond well to selective serotonin reuptake inhibitor (SSRI) antidepressants, while those with elevated blood levels of CRP seem to respond better to a dopamine-active antidepressant such as bupropion or a noradrenergic-active antidepressant such as nortriptyline or the serotonin norepinephrine reuptake inhibitor (SNRI) antidepressant duloxetine. Patients with high inflammation at baseline also seem to respond better to intravenous ketamine and oral doses of omega-3 fatty acids.
Studies of animals have suggested that inflammation throughout the body is implicated in depression. Studies in which rodents are repeatedly defeated by larger animals show that these animals have increased inflammation from lymphocites (a type of white blood cells) in the blood, and monocytes (another type of white blood cells) from the bone marrow and spleen. This inflammation can induce depression-like behaviors in the rodents, which is prevented if the inflammatory mechanisms are blocked. These data suggest that depression is not just in the brain—inflammation from all over the body plays an important role.
Read more
How Stress Triggers Inflammation and Depression
 Depression and bipolar disorder are associated with increases in markers of inflammation that can be found in the brain and blood. It is increasingly clear that the mechanisms that cause depression are not just in the brain, but actually throughout the body. These include two signaling systems that begin in the bone marrow and the spleen.
Depression and bipolar disorder are associated with increases in markers of inflammation that can be found in the brain and blood. It is increasingly clear that the mechanisms that cause depression are not just in the brain, but actually throughout the body. These include two signaling systems that begin in the bone marrow and the spleen.
When a small mouse is repeated defeated by a larger animal, they show depression-like symptoms known as defeat stress. Animal studies have shown that stress and danger signals are perceived and relayed to the amygdala and the hypothalamus. The sympathetic nervous system releases the neurotransmitter norepinephrine into bone marrow, where stem cells are turned into activated monocytes (a type of white blood cells) that are then released into the blood. The monocytes travel to the brain, leading to the activation of more inflammatory cells.
Blocking part of this process can prevent the depression-like behaviors from occurring. If the bone marrow monocytes are blocked from entering the brain, inflammation and defeat stress behaviors like social avoidance do not occur. However, if there is a second bout of defeat stress, primed monocytes that have been stored in the spleen are released and travel to the brain, producing further increases in inflammatory cells and even more defeat stress behaviors.
If these monocytes from the spleen are blocked, the inflammation and the reaction to the new stressor do not occur.
Stress also activates lymphocytes (another type of white blood cells) to secrete the inflammatory cells Il-6. If this Il-6 secretion is inhibited, defeat stress behaviors can be prevented.
Defeat stress also leads to the release of the neurotransmitter glutamate. Some of this cascade begins in the brain, which evaluates stressors and releases IL-1 beta, another type of inflammatory cell. It slows down the production of glutamate, while IL-6 can endanger neurons and is associated with anhedonia—loss of interest in pleasurable activities. This cascade also leads to the production of another type of inflammatory cell, TNF-alpha, which has adverse effects on biochemistry, brain, and behavior.
This understanding of the role of the brain and body provides new targets for drug development. If inflammatory processes are blocked, defeat stress behaviors do not occur. Researcher Michael D. Weber and colleagues described this process in detail in the journal Neuropsychopharmacology Reviews in 2017.
Together these observations suggest that inflammatory processes in the body are crucial to the development of some stress- and inflammation-related depressive behaviors.
Measuring Inflammation in Neuropsychiatric Disorders May Shed Light on Treatment
Meta-analyses have found links between elevated levels of inflammatory markers and many neuropsychiatric disorders, including depression, bipolar disorder, schizophrenia, post-traumatic stress disorder (PTSD) and traumatic brain injury. Multiple studies also show that those with elevated inflammatory markers such as interleukin-1, interleukin-6, tumor necrosis factor (TNF) alpha, and c-reactive protein (CRP) also respond less well to typical treatments than those with normal levels of these markers in the blood.
These links suggest that it could be useful to measure levels of these inflammatory markers in the blood of people who are responding poorly to medications. If one or more of these markers are elevated, it might be a sign that treatment with an anti-inflammatory agent could be helpful. Preliminary studies have shown that some neuropsychiatric disorders may improve after treatment with anti-inflammatory drugs such as aspirin, celecoxib (Celebrex), and the antibiotic minocycline, among others.
Arthritis Drug Celecoxib May Improve Bipolar Depression When Paired with Escitalopram
A new study suggests that for people with bipolar depression, the anti-inflammatory drug celecoxib (Celebrex), typically used to treat arthritis, can boost the effectiveness of the antidepressant escitalopram (Lexapro).
In the 8-week study by researcher Angelos Halaris and colleagues, adults with bipolar depression were randomly assigned to one of two groups. The first group received the selective-serotonin reuptake inhibitor (SSRI) antidepressant escitalopram plus celecoxib to target inflammation. The second group received just the antidepressant escitalopram and a placebo.
By the end of the study, 78% of the group taking the anti-arthritis drug had seen major improvement in their depression, with 63% reporting that it had lifted completely. Meanwhile in the placebo group, only 45% reported major improvement, and 10% reported remission.
The group that received celecoxib with their escitalopram also began seeing improvement within one week of beginning treatment, instead of after four to six weeks, which is typical of antidepressant treatment.
Researchers think depression creates an immune response leading to chronic inflammation, which can upset the balance of neurotransmitters in the brain and make antidepressants less effective. Halaris suggests that reducing this inflammation with a drug like celecoxib can make antidepressants more effective.
The research was presented at the Fifth International Congress on Psychiatry and the Neurosciences and has not yet been published.
Inflammatory Marker CRP Higher in Bipolar Disorder, Particularly Mania
Inflammation has been linked to mood disorders. A 2016 meta-analysis in the journal Lancet Psychiatry described the role of inflammatory marker C-reactive protein (CRP) in bipolar episodes. Researchers led by Brisa S. Fernandes identified 27 studies that measured CRP levels in a total of 2,161 patients with bipolar disorder and 81,932 healthy participants. The researchers determined that compared to healthy controls, people with bipolar disorder have higher levels of CRP. CRP levels were moderately elevated between episodes and during depression, and substantially elevated during episodes of mania.
Editor’s Note: This meta-analysis shows that CRP is linked to bipolar disorder, and the inflammatory burden is highest during mania. It remains to be seen whether anti-inflammatory treatments work best in patients with high CRP levels compared to normal CRP levels.
CRP is also a risk factor for cardiovascular disease, and lithium and other treatments for bipolar disorder probably lower CRP levels.
The same group of researchers previously showed that statins, drugs typically used to lower cholesterol, could help alleviate depression. Since statins have anti-inflammatory effects, they can probably reduce depression risk in addition to lowering cardiovascular risk, as initial studies suggest.
Other drugs with anti-inflammatory effects that may improve depression include the anti-arthritis drug celecoxib and the antibiotic minocycline. The amino acid N-acetylcysteine and omega-3 fatty acids also have anti-inflammatory effects and have been found to improve depression in some studies.
Anti-Inflammatory Treatments Improve Depression
Inflammation can interfere with the balance of neurotransmitters in the brain, making antidepressants less effective. Anti-inflammatory treatments (such as those used to treat rheumatoid arthritis) may help. In a 2016 meta-analysis published in the journal Molecular Psychiatry, researchers led by Nils Kappelmann analyzed the results of 20 clinical trials of chronic inflammatory conditions where depressive symptoms were also recorded. In a subset of 7 clinical trials that compared anti-inflammatory treatment to placebo, they found that anti-inflammatory treatment improved depressive symptoms significantly compared to placebo.
The anti-inflammatory drugs studied most often targeted the inflammatory marker tumor necrosis factor (TNF) alpha using an antibody. Some of the anti-inflammatory drugs that improved depressive symptoms were adalimumab, etanercept, infliximab, and tocilizumab.
The researchers also found that those participants with the most inflammation when they began treatment saw the largest improvement in their depression after taking anti-inflammatory treatments.
Kappelmann and colleagues suggest that inflammation may cause depression, and that anti-inflammatory drugs may be useful in the treatment of depression in people with high inflammation.
Preventative Treatment Should Begin After First Manic Episode
Evidence from multiple studies has indicated the importance of beginning preventative treatment, particularly with lithium, early in the course of bipolar disorder. A 2016 comprehensive literature review by researcher Katie Joyce and colleagues in the International Journal of Bipolar Disorders concluded that psychoeducation and medication are more effective in bipolar disorder when applied in earlier stages of the illness rather than later stages.
Several studies suggest that treatment for bipolar disorder should be started specifically after the first manic episode.
A 2014 study by researcher Lars Kessing and colleagues in the British Journal of Psychiatry examined 4,700 patients treated with lithium in Denmark. Kessing and colleagues found that those who started treatment after one manic episode were less likely to find lithium ineffective than those who started later.
Another study by researcher Michael Berk and colleagues presented at the International Society for Bipolar Disorders found that after a first manic episode, a year of treatment with lithium was much more effective on all measures of outcome, including mania and depression ratings, brain imaging, and neuropsychological functioning, than a year when patients were randomized to quetiapine (Seroquel).
Researcher Lakshmi Yatham and colleagues presented research at the International Society for Bipolar Disorders showing that patients recovered from the neuropsychological deficits associated with a first episode of mania if they were well treated and had no further episodes, while those who had new episodes did not return to their baseline capabilities. This suggests that early treatment that prevents future episodes helps maintain a healthy brain.
Kessing and colleagues previously reported in the British Journal of Psychiatry in 2013 that patients randomized to two years of treatment in an outpatient clinic specializing in mood disorders following a first hospitalization for mania had 40% fewer recurrences of bipolar episodes over the next six years than those who received treatment as usual. These data indicate that early treatment, which may include psychotherapy, medications, mood charting (i.e. keeping a daily record of symptoms) and illness education, can improve the long-term course of illness. Lithium is often a key component of such treatment.
Editor’s Note: This type of intensive, ongoing treatment is not the norm after a first manic hospitalization in the United States, but it should be. Given the new data on the impact of starting lithium after a first episode of mania, and lithium’s superiority over quetiapine in the year following a first episode, lithium treatment should be standard following a diagnosis of bipolar disorder.
In the past, sometimes doctors have recommended waiting until a patient has had multiple episodes of mania before beginning preventative treatment with lithium. This now appears to be a mistake.
Lithium protects against depressive as well as manic recurrences, and there is also evidence that it increases hippocampal and cortical volume, helping prevent cognitive deterioration in those with mild cognitive impairment. Lithium is also the most effective mood stabilizer for preventing suicide, and it increases the length of telomeres (caps on the end of DNA strands), thus preventing a wide range of medical and psychiatric illnesses. Lithium may need to be combined with other drugs to achieve a complete remission, but using it after a first mania is a good place to start.