Fear Memories Can Be Erased or Provoked in Animals
Researchers have identified neurons responsible for remembering conditioned fear in the amygdala of rodents, and can turn them on and off. At the 2013 meeting of the Society of Biological Psychiatry, Sheena A. Josselyn gave a breath-taking presentation on this process.
When animals hear a tone they have learned to associate with the imminent delivery of a shock in a given environment, they learn to avoid that environment, and they reveal their learning of the tone-shock association by freezing in place. Josselyn was able to observe that 20% of the neurons in the lateral nucleus of the amygdala were involved in this memory trace. They were revealed by their ability to increase the transcription factor CREB, which is a marker of cell activation. Using cutting-edge molecular genetic techniques, the researchers could selectively eliminate only these CREB-expressing neurons (using a new technology in which a diphtheria toxin is attached to designer receptors exclusively activated by designer drugs, or DREADDs) and consequently erase the fear memory.
The researchers could also temporarily inhibit the memory, by de-activating the memory trace cells, or induce the memory, so that the animal would freeze in a new context. Josselyn and colleagues were able to identify the memory trace for two different tones in two different populations of amygdala neurons.
The same molecular tricks with memory also worked with cocaine cues, using what is known as a conditioned place preference test. A rodent will show a preference for an environment where it received cocaine. Knocking out the selected neurons would remove the memory of the cocaine experience, erasing the place preference.
The memory for cocaine involved a subset of amygdala neurons that were also involved in the conditioned fear memory trace. Incidentally, Josselyn and her group were eventually able to show that amygdala neurons were in competition with each other as to whether they would be involved in the memory trace for conditioned fear or for the conditioned cocaine place preference.
Poverty Impacts Brain Development
 In a 2013 study of children by Luby et al. in the Journal of the American Medical Association Pediatrics, poverty in early childhood was associated with smaller white and gray matter in the cortex and with smaller volume of the amygdala and hippocampus when the children reached school age. The effects of poverty on hippocampal volume were mediated by whether the children experienced stressful life events and whether a caregiver was supportive or hostile.
In a 2013 study of children by Luby et al. in the Journal of the American Medical Association Pediatrics, poverty in early childhood was associated with smaller white and gray matter in the cortex and with smaller volume of the amygdala and hippocampus when the children reached school age. The effects of poverty on hippocampal volume were mediated by whether the children experienced stressful life events and whether a caregiver was supportive or hostile.
The children were recruited from primary care and day care settings between the ages of three and six, and were studied for five to ten years. They were initially assessed annually for three to six years and information on psychosocial, behavioral, and developmental dimensions were collected. Then the children took part in a magnetic resonance imaging (MRI) scan and continued annual assessments that included information such as whether the children experienced stressful life events.
Previous research has shown that poverty affects children’s psychosocial development and economic success in adulthood. This research shows that poverty also affects brain development. The findings suggest important targets for intervention that could help prevent these developmental deficits.
Selective Erasure Of Cocaine Memories In A Subset Of Amygdala Neurons
We have written before about the link between memory and both fear and addiction. At a recent scientific meeting, researchers discussed attempts to erase cocaine-cue memories in mice.
In articles published in Science in 2007 and 2009, Han et al. showed that about 20% of neurons in the lateral amygdala of mice were involved in the formation of a fear memory, and that selective deletion of these neurons could erase the fear memory. Using the same methodology, Josh Sullivan et al. identified neurons that were active in the mouse brain during cocaine conditioning. Amygdala activity showed that the mice preferred an environment where they received cocaine to an environment where they didn’t. The researchers noticed increased cyclic AMP, a messenger that led to increased production of calcium responsive element binding protein (CREB). When the researchers targeted the neurons in the lateral amygdala that were overexpressing CREB, they found that selective destruction of the overexpressing neurons disrupted the cocaine-induced place preference.
The research team further documented this effect by temporarily, rather than permanently, knocking out neuronal function. They could reversibly turn off neurons with an inert compound that promotes neuronal inhibition. Silencing the neurons that were overexpressing CREB before the conditioned place preference testing also limited cocaine-induced place preference memory.
Editor’s Note: While this type of intervention is not feasible in humans with cocaine addiction, these data do shed more light on the mechanisms behind cocaine conditioning.
We have written before that if extinction training to break a cocaine habit or neutralize a learned fear is performed within the brain’s memory reconsolidation window (five minutes to one hour after memory recall), it can induce long-lasting alterations in cocaine craving or conditioned fear.
It is possible that properly timed extinction of cocaine- or fear-conditioned memories might work similarly to the selective silencing of neurons that was carried out in the mice using a drug that inhibited CREB-activated neurons. Determining the commonalities between these ways of eliminating conditioned memories could lead to a whole new set of psychotherapeutic approaches to anxiety disorder, addictions, and other pathological habits.
The Amygdala Plays a Role in Habitual Cocaine Seeking
 At a recent scientific meeting, Jennifer E. Murray et al. presented findings about the amygdala’s role in habitual cocaine seeking. The amygdala is the part of the brain that makes associations between a stimulus and a response. When a person begins using cocaine, a signal between the amygdala and the ventral striatum (also known as the nucleus accumbens), the brain’s reward center, creates a pleasurable feeling for the person. The researchers found that in mice who have learned to self-administer cocaine, as an animal progresses from intermittent use to habitual use, the amygdala connections shift away from the ventral striatum toward the dorsal striatum, a site for motor and habit memory. If amygdala connections to the dorsal striatum are severed, the pattern of compulsive cocaine abuse does not develop.
At a recent scientific meeting, Jennifer E. Murray et al. presented findings about the amygdala’s role in habitual cocaine seeking. The amygdala is the part of the brain that makes associations between a stimulus and a response. When a person begins using cocaine, a signal between the amygdala and the ventral striatum (also known as the nucleus accumbens), the brain’s reward center, creates a pleasurable feeling for the person. The researchers found that in mice who have learned to self-administer cocaine, as an animal progresses from intermittent use to habitual use, the amygdala connections shift away from the ventral striatum toward the dorsal striatum, a site for motor and habit memory. If amygdala connections to the dorsal striatum are severed, the pattern of compulsive cocaine abuse does not develop.
Editor’s Note: These data indicate that the amygdala is involved in cocaine-related habit memory, and that the path of activity shifts from the ventral to the dorsal striatum as the cocaine use becomes more habit-based—automatic, compulsive, and outside of the user’s awareness.
As we’ve reviewed before, the amygdala also plays a role in context-dependent fear memories, such as those that occur in post-traumatic stress disorder (PTSD). The process of retraining a person with PTSD to view a stimulus without experiencing fear is called extinction training. A study by Agren et al. published in Science in 2012 demonstrated that when extinction training of a learned fear took place within the brain’s memory reconsolidation window (five minutes to one hour after active memory recall), the training was sufficient to not only “erase the conditioned fear memory trace in the amygdala, but also decrease autonomic evidence of fear as revealed in skin conductance changes in volunteers.”
The preclinical data presented by Murray and colleagues suggest the possibility that amygdala-based habit memory traces could also be revealed via functional magnetic resonance imaging (fMRI) in subjects with cocaine addiction. Attempts at extinction of cocaine craving, if administered within the memory reconsolidation window, might likewise be able to erase the cocaine addiction/craving memory trace, as Xue et al. reported in Science in 2012.
Amygdala Size Linked to Manic Symptom Severity
 In two posters presented at the 2012 meeting of the American Academy of Child and Adolescent Psychiatry, a research group led by Kiki Chang reported that increased severity of manic symptoms is associated with increased size of the amygdala (especially the right amygdala) in adolescents who are at high risk for developing bipolar disorder.
In two posters presented at the 2012 meeting of the American Academy of Child and Adolescent Psychiatry, a research group led by Kiki Chang reported that increased severity of manic symptoms is associated with increased size of the amygdala (especially the right amygdala) in adolescents who are at high risk for developing bipolar disorder.
The amygdala is a crucial area for emotion regulation. The increasing size, either with more manic symptoms or as patients with bipolar disorder age into adulthood compared to normal volunteer controls (as we describe in the article on brain imaging at far left) could reflect increased use of the amygdala in bipolar disorder.
The increased amygdala size could be linked to increased emotion dysregulation, or it could be a compensatory mechanism in which the amygdala works harder to exert better emotion control.
Experience-dependent neuroplasticity describes a phenomenon in which the volume of a brain area increases as it gets more use (like a muscle that grows when it gets more exercise). One interesting example in which this may occur is London taxi drivers, who have larger hippocampi than the general public. (The hippocampus is responsible for some of the brain’s spatial recognition abilities.) This could be explained in two different ways. The discrepancy in size between the hippocampi of taxi drivers and of the general population may exist because the taxi drivers’ brains change over the course of their careers via experience-dependent neuroplasticity, or it may exist because those with excellent spatial recognition abilities and bigger hippocampi choose to become taxi drivers.
How Illness Progresses In The Recurrent Affective Disorders
This editor (RM Post) in collaboration with Jacqueline Fleming and Flavio Kapczinski published the article “Neurobiological mechanisms of illness progression in the recurrent affective disorders” in the Journal of Psychiatric Research this year. The article built on several themes about the progression of bipolar illness that had been explored in previous research.
These themes include:
- The likely acceleration of repeated episodes as a function of the number of prior episodes (episode sensitization)
- The increased responsivity of the illness to repeated stressors (stress sensitization)
- The increased behavioral reactivity to repeated use of psychomotor stimulants such as cocaine (stimulant-induced behavioral sensitization)
Not only are these observations well documented in the scientific literature, but recent observations also suggest that each type of sensitization can show cross-sensitization to the other two types. That is, individuals exposed to repeated stressors are more likely both to experience affective illness episodes and to adopt comorbid substance abuse. In a similar way, episodes of an affective disorder and stressors may also be associated with the relapse into drug administration in those who have been abstinent.
In addition to these mechanisms of illness progression in the recurrent affective disorders, the new article reviews the literature showing that the number of affective episodes or the duration of the illness appear to be associated with a variety of other clinical and neurobiological variables.
The number of affective episodes a patient experiences is associated with the degree of cognitive dysfunction present in their bipolar illness, and experiencing more than 4 episodes of unipolar or bipolar depression is a risk factor for dementia in late life. A relative lack of response to most treatments is also correlated with the number of prior episodes, and this holds true for response to naturalistic treatment in general. While most of these data are correlational and the direction of causality cannot be ascertained for certain, it is likely that the number of affective episodes and/or their duration could account for and drive difficulties with treatment and with cognitive function.
If this were the case, one would expect to see a variety of neurobiological correlates with the number of prior episodes or duration of illness, and in the article we summarize those that have been found in unipolar and bipolar disorder. Considerable data indicate that cortical volume and degrees of prefrontal cortical dysfunction can vary as a function of number of prior episodes. There is evidence that increased activity of the amygdala and the nucleus accumbens are also related to episodes or duration of illness. In those with unipolar depression, the volume of the hippocampus is decreased with longer duration of illness. Read more
Opening The Reconsolidation Window to Extinguish Fear Memories: A New Conceptual Approach to Psychotherapeutics
 Memory processes occur in several phases. Short-term memory is converted to long-term memory by a process of consolidation that requires the synthesis of new proteins. Transcription factors in the nuclei of hundreds of millions of nerve cells are activated so that specific synapses can be modified for the long term. If protein synthesis is inhibited during a period within a few hours after new learning has occurred, what was learned never gets consolidated and is essentially forgotten. It is thought that this phase of consolidation happens when a memory trace moves from short-term storage in the hippocampus to long-term storage in the cerebral cortex.
Memory processes occur in several phases. Short-term memory is converted to long-term memory by a process of consolidation that requires the synthesis of new proteins. Transcription factors in the nuclei of hundreds of millions of nerve cells are activated so that specific synapses can be modified for the long term. If protein synthesis is inhibited during a period within a few hours after new learning has occurred, what was learned never gets consolidated and is essentially forgotten. It is thought that this phase of consolidation happens when a memory trace moves from short-term storage in the hippocampus to long-term storage in the cerebral cortex.
Recently a later phase of memory storage called reconsolidation has been identified. When an old memory is recalled, the reconsolidation window opens, and the memory trace becomes temporarily amenable to change. The reconsolidation window (the period during which the trace can be revised) is thought to begin five minutes after a memory is recalled and last for an hour or possibly two. New learning that takes place during the reconsolidation window can be more profound than learning that occurs without recall of the related memory or after the reconsolidation window has closed.
Consider the example of a fearful memory created when a person is attacked in a dark alley. If the person repeatedly visits the same alley without being attacked, they can eventually become less afraid of dark places. Repeated viewing of pictures of dark places can also extinguish the fear. These are typical ways in which a fear memory is extinguished. However, the original fear is subject to spontaneous recovery (the fear of dark places returns without provocation) or to reactivation (if another dangerous situation is encountered, the person may regain their fear of dark alleys).
The new findings suggest that if the extinction process (the repeated exposures to the pictures of the dark alley) takes place during the reconsolidation window after the fear memory of being attacked is recalled, the old fear can be permanently reversed (wiped clean, or re-edited such that it appears forgotten) so that it is no longer subject to spontaneous recovery or reactivation.
Editor’s Note: To accomplish extinction training within the reconsolidation window, first a person must actively recall the old memory, opening the reconsolidation window. Then, after a 5-minute delay, extinction training (e.g. new learning that the old feared place is now safe) should take place within the next hour. This process has been demonstrated in animal studies and is thought to be clinically relevant for humans in the case of phobic anxiety and post-traumatic stress disorder (PTSD). The psychotherapeutic implications of using the reconsolidation window to better ameliorate PTSD fears, avoidance, and flashbacks are enormous.
Observing the Amygdala’s Role in the Extinction of Fear Memory Traces
The amygdala is a crucial part of the learned or conditioned fear pathway. It is activated during fear conditioning and during the recall of cues associated with the fear experience. If the amygdala is removed, conditioning fear does not occur.
A new study published in Science this year by Agren et al. indicates that in humans, the amygdala-based response to conditioned fear can be completely abolished using extinction training within the memory reconsolidation window. Training that took place 10 minutes after the fear memory was activated was successful, while training that took place 6 hours later was not. Read more
Cortex Shrinks and Amygdala Grows in Childhood Bipolar Disorder
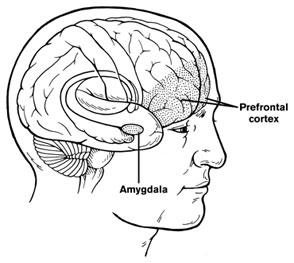 At a symposium on new research on juvenile bipolar disorder at the meeting of the American Academy of Child and Adolescent Psychiatry (AACAP) in 2010, the discussant Kiki Chang of Stanford University reported some recent neurobiological findings on childhood bipolar disorder. He found evidence that prefrontal cortical volume appears to decrease over the course of the illness and, conversely, there was evidence of increases in amygdala volume. He also found that the volume of the striatum (or caudate nucleus, which is involved in motor control) increased in children with bipolar illness or bipolar illness comorbid with ADHD, but decreased in children with ADHD alone.
At a symposium on new research on juvenile bipolar disorder at the meeting of the American Academy of Child and Adolescent Psychiatry (AACAP) in 2010, the discussant Kiki Chang of Stanford University reported some recent neurobiological findings on childhood bipolar disorder. He found evidence that prefrontal cortical volume appears to decrease over the course of the illness and, conversely, there was evidence of increases in amygdala volume. He also found that the volume of the striatum (or caudate nucleus, which is involved in motor control) increased in children with bipolar illness or bipolar illness comorbid with ADHD, but decreased in children with ADHD alone.
Chang cited the study of Singh et al. (2010) who found that the subgenual anterior cingulate volume early in the course of illness was smaller in adolescent-onset bipolar disorder compared to controls. Given this evidence of prefrontal cortical and anterior cingulate deficits, Dr. Chang raised the possibility that treatment with lithium and other agents with potential neurotrophic and neuroprotective effects might be able to prevent these neurobiological aspects of illness progression in young patients.




