Only Fluoxetine is More Effective Than Placebo for Children and Adolescents with Depression
 In a meta-analysis published in 2016, researchers Andrea Cipriani, Xinyu Zhou, and colleagues reported that many antidepressants are not effective in children and adolescents. Fluoxetine alone was more effective than placebo. Other antidepressants also caused high study drop-out rates compared to placebo.
In a meta-analysis published in 2016, researchers Andrea Cipriani, Xinyu Zhou, and colleagues reported that many antidepressants are not effective in children and adolescents. Fluoxetine alone was more effective than placebo. Other antidepressants also caused high study drop-out rates compared to placebo.
In an article published in the journal The Lancet, Cipriani, Zhou, and colleagues analyzed 34 randomized, controlled clinical trials of antidepressants in children and adolescents. These trials included a total of 5,260 participants and 14 different antidepressants.
The researchers determined that much of the evidence was of a low quality. Only fluoxetine was statistically significantly more effective than placebo. Fluoxetine was also more tolerable to patients than duloxetine or imipramine. Patients who received imipramine, venlafaxine, or duloxetine were more likely to drop out of studies due to adverse events compared to patients who received placebo.
The authors suggest that prescribing antidepressants to children or adolescents may not necessarily be beneficial, and that fluoxetine is probably the best option to consider.
Editor’s Note: It may be best to use caution when prescribing antidepressants to children or adolescents. First, these data that suggest that many antidepressants are ineffective in young people. In addition, depression in children and adolescents may be a sign of bipolar disorder, and antidepressant use may cause activation or switching into mania in vulnerable patients.
While there is a warning about using antidepressants in young people because of the risk of increased suicidal ideation, the actual suicide rate in young populations decreases when these patients take antidepressants and cognitive behavioral therapy. Psychotherapy should be a high priority. Other safe adjunctive approaches might include omega-3 fatty acids, N-acetylcysteine, vitamin D3, and folic acid. Evidence for the efficacy of rTMS in young people is also positive and growing.
Meta-Analysis Suggests Statins Can Help Treat Depression
Depression and poor cardiovascular health often go hand in hand. Now it seems a treatment for high cholesterol may also help treat depression. A 2016 study by E. Salagre and colleagues in the Journal of Affective Disorders analyzed evidence from 3 earlier studies of drugs called statins, which inhibit an enzyme needed for the production of cholesterol, for people with depression.
The three studies included a total of 165 participants taking an antidepressant (citalopram or fluoxetine) for moderate or severe depression. Of these participants, 82 were prescribed an additional statin (lovastatin, atorvastatin, or simvastatin), while the remaining were given a placebo. After 6 to 12 weeks, those who had received a statin reported greater improvement in their depression than those who had received a placebo in addition to the antidepressant. No serious side effects were reported.
These data are also consistent with other studies showing that women on statins had fewer depressions over subsequent years than those not taking statins.
Thiamine (Vitamin B1) May Increase Effectiveness of Antidepressants
A new study suggests that the nutritional supplement vitamin B1, also known as thiamine, can improve symptoms of depression when taken with an antidepressant. Edith Holsboer-Trachsler and colleagues presented the research from their randomized, double-blind, placebo-controlled study at a recent scientific meeting. In a 12-week study, about 50 adults (averaging 35 years of age) with major depression were prescribed a selective-serotonin reuptake inhibitor (SSRI) antidepressant. In addition, half received thiamine supplements while the other half were given placebos. Starting at six weeks, those receiving thiamine with their antidepressant showed more improvement in their depressive symptoms than those receiving the antidepressant alone.
Thiamine is an essential nutrient for humans. It is found in foods such as yeast, pork, cereal grains, and certain vegetables. Thiamine deficiency has been linked to irritability and symptoms of depression, while thiamine supplementation can improve mood and reduce feelings of stress. No side effects were reported in the study.
Holsboer-Trachsler and colleagues hope that thiamine supplementation may help patients adhere to their antidepressant regimens by decreasing the time it takes until their moods begin to lift.
Treating Prenatal Depression Improves Outcomes for Mothers and Babies
 A recent study confirms that women who are depressed during pregnancy are more likely to experience adverse pregnancy outcomes such as preterm or cesaerean delivery and small or underweight babies. However, antidepressant treatment improved outcomes for pregnant women with depression.
A recent study confirms that women who are depressed during pregnancy are more likely to experience adverse pregnancy outcomes such as preterm or cesaerean delivery and small or underweight babies. However, antidepressant treatment improved outcomes for pregnant women with depression.
The 2016 study by Kartik K. Venkatesh and colleagues in the journal Obstetrics & Gynecology included 7,267 women who gave birth after at least 20 weeks of pregnancy. About 11% of the women screened positive for depression during their pregnancy. Depressed mothers-to-be were more likely to give birth before 37 weeks and before 32 weeks compared to nondepressed mothers-to-be. The depressed women were also more likely to deliver small babies or babies weighing under 2500g.
About 7% of the women in the study received antidepressant medication. Compared to nondepressed women, the women taking antidepressants did not have greater rates of early delivery or small babies. However, the authors caution that because so few women received antidepressants, the study does not reveal whether antidepressants improve outcomes for depressed pregnant women.
Most SSRIs Free of Birth Defect Risk Early in Pregnancy, Fluoxetine and Paroxetine are Exceptions
A large study of women who took selective serotonin reuptake inhibitor (SSRI) antidepressants in the month before pregnancy and throughout the first trimester suggests that there is a smaller risk of birth defects associated with SSRI use than previously thought, though some risks were elevated in women who took paroxetine or fluoxetine.
The 2015 study, by Jennita Reefhuis and colleagues in the journal BMJ, investigated the drugs citalopram, escitalopram, fluoxetine, paroxetine, and sertraline, and examined birth defects that had previously been associated with SSRI use in smaller studies. The participants were 17,952 mothers of infants with birth defects and 9,857 mothers of infants without birth defects who had delivered between 1997 and 2009.
Sertraline was the most commonly used SSRI among the women in the study. None of the birth defects included in the study were associated with sertraline use early in pregnancy. The study found that some birth defects were 2 to 3.5 times more likely to occur in women who had taken fluoxetine or paroxetine early in their pregnancies.
Five different birth defects, while uncommon, were statistically linked to paroxetine use: anencephaly (undersized brain), heart problems including atrial septal defects and right ventricular outflow tract obstruction defects, and defects in the abdominal wall including gastroschisis and omphalocele. Two types of birth defects were associated with fluoxetine use: right ventricular outflow tract obstruction defects and craniosynostosis (premature fusion of the skull bones). Absolute incidence of these defects was also low.
Another Antidepressant Fails in Bipolar Depression
Despite repeated studies, including meta-analyses, showing that antidepressants that work in unipolar depression do not work in bipolar depression as adjuncts to mood stabilizers, antidepressants remain widely used for the treatment of bipolar depression. A recent study of the antidepressant agomelatine has shown that it is not effective in bipolar depression. In patients taking lithium or valproate but still depressed, agomelatine was no better than placebo at reducing depression.
Agomelatine has an unusual mechanism of action (blockade of 5HT-2C receptors and activation of melatonin M1 and M2 receptors) that helps normalize sleep and circadian rhythms, but only in unipolar depression. Until this study by Lakshmi Yatham and colleagues in the British Journal of Psychiatry, it was thought that these properties would make the drug ideal for bipolar depression.
Three atypical antipsychotics are have been approved by the Federal Drug Administration for bipolar depression: quetiapine (Seroquel), lurasidone (Latuda), and the olanzepine-fluoxetine combination Symbyax. These, used alongside mood stabilizers (lithium, valproate, carbamazepine, and lamotrigine) are more effective treatments for bipolar depression. There are other adjunctive treatments that may be helpful, such as the antioxidant N-acetylcysteine, vitamin D3, and folate.
Genetic Variation Predicts Which Type of Antidepressant Will Be Effective
 In a six-month study of Caucasian patients, normal variations in the gene that is responsible for brain-derived neurotrophic factor (BDNF) predicted whether patients would respond better to a selective serotonin reuptake inhibitor (SSRI) antidepressant versus a serotonin and norepinephrine reuptake inhibitor (SNRI) or a tricycle antidepressant. There are several common variants of the BDNF gene, depending on which types of amino acids appear in its coding—valine or methionine. Patients with the most common version, two valines (or Val66Val), responded better to SSRIs. About two-thirds of the population has this version of the gene, which functions most efficiently. The remaining third have at least one methionine in the BDNF gene. Patients with a Met variation responded better to SNRIs and tricyclic antidepressants.
In a six-month study of Caucasian patients, normal variations in the gene that is responsible for brain-derived neurotrophic factor (BDNF) predicted whether patients would respond better to a selective serotonin reuptake inhibitor (SSRI) antidepressant versus a serotonin and norepinephrine reuptake inhibitor (SNRI) or a tricycle antidepressant. There are several common variants of the BDNF gene, depending on which types of amino acids appear in its coding—valine or methionine. Patients with the most common version, two valines (or Val66Val), responded better to SSRIs. About two-thirds of the population has this version of the gene, which functions most efficiently. The remaining third have at least one methionine in the BDNF gene. Patients with a Met variation responded better to SNRIs and tricyclic antidepressants.
The study by R. Colle and colleagues was published in the Journal of Affective Disorders in 2015. Of the patients who were prescribed SSRIs, 68.1% of patients with the Val/Val version responded to the medication after three months, compared to 44% of the patients with a Met version. Of patients prescribed SNRIs or tricyclics, 60.9% of the Met patients reached remission by six months, compared to only 33.3% of the Val/Val patients.
Editor’s Note: In an earlier BNN we reported that according to research published by Gonzalo Laje and colleagues in the journal Biological Psychiatry in 2012, depressed patients with the better functioning Val66Val allele of BDNF respond best to ketamine, while those with the intermediate functioning Val66Met allele respond less well.
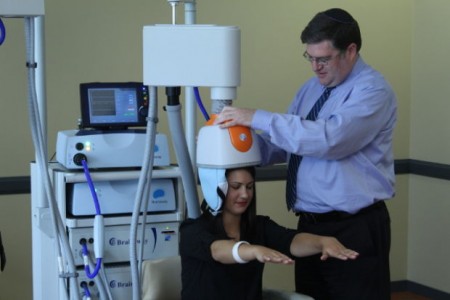
Deep Transcranial Magnetic Stimulation Safe and Effective in Major Depression
Repeated transcranial magnetic stimulation is a non-invasive procedure that has been approved for the treatment of severe depression since 2008. In rTMS treatment, a figure-8–shaped electromagnetic coil is placed against the forehead and magnetic pulses that can penetrate the scalp are converted into small electrical currents that stimulate neurons in the brain up to 1.5 cm deep. More recently, in 2013, the Federal Drug Administration approved a device with an H-shaped coil that delivers deep transcranial magnetic stimulation (dTMS). It can stimulate a wider area, and up to 8 cm deep.
Y. Levkovitz and colleagues have published the first double-blind randomized controlled multicenter trial of dTMS, reporting in the journal World Psychiatry that the intervention was effective and safe in patients who had not responded to antidepressant medication.
The study included 212 patients aged 22–68 years. All participants had failed to respond to one to four antidepressants or had not been able to tolerate the side effects of at least two antidepressants during their current episode of depression. The patients were randomized to receive either a sham treatment or 18 Hz dTMS over the prefrontal cortex acutely for four weeks and biweekly for 12 weeks for a total of 20 sessions.
The patients who received dTMS showed significantly greater improvement in symptoms than those who received the sham treatment, with a moderately large effect size of 0.76. Response and remission rates were also better in those who received dTMS. Response rates were 38.4% for the dTMS group versus 21.4% in the sham group. Remission rates were 32.6% for the dTMS group and 14.6% for the sham group. These difference in response remained stable during the three months of the study.
Side effects were minor except for a seizure that occurred when the protocol for the treatment was breached.
Rapid-Onset Antidepressant Treatments
At the International College of Neuropsychopharmacology (CINP) World Congress of Neuropsychopharmacology in 2014, several presentations and posters discussed treatments that bring about rapid-onset antidepressant effects, including ketamine, isoflurane, sleep deprivation, and scopolamine.
Ketamine’s Effects
Multiple studies, now including more than 23 according to researcher William “Biff” Bunney, continue to show the rapid-onset antidepressant efficacy of intravenous ketamine, usually at doses of 0.5 mg/kg over 40 minutes. Response rates are usually in the range of 50–70%, and effects are seen within two hours and last several days to one week. Even more remarkable are the six studies (two double-blind) reporting rapid onset of antisuicidal effects, often within 40 minutes and lasting a week or more. These have used the same doses or lower doses of 0.1 to 0.2mg/kg over a shorter time period.
Attempts to sustain the initial antidepressant effects include repeated ketamine infusions every other day up to a total of six infusions, a regimen in which typically there is no loss of effectiveness. Researcher Ronald Duman is running a trial of co-treatment with ketamine and lithium, since both drugs block the effects of GSK-3, a kinase enzyme that regulates an array of cellular functions, and in animals the two drugs show additive antidepressant effects. In addition, lithium has been shown to extend the acute antidepressant effects of one night of sleep deprivation, which are otherwise reversed by a night of recovery sleep.
Ketamine’s effects are related to the neurotransmitter glutamate, for which there are several types of receptors, including NMDA and AMPA. Ketamine causes a large burst of glutamate presumably because it blocks NMDA glutamate receptors on inhibitory interneurons that use the neurotransmitter GABA, causing glutamatergic cells to lose their inhibitory input and fire faster. While ketamine blocks the effects of this glutamate release at NMDA receptors, actions at AMPA receptors are not blocked, and AMPA activity actually increases. This increases brain-derived neurotrophic factor (BDNF), which is also required for the antidepressant effects of ketamine. Ketamine also increases the effects of mTOR, a kinase enzyme that regulates cell growth and survival, and if these are blocked with the antibiotic rapamycin, antidepressant effects do not occur.
In animal studies, ketamine increases dendritic spine growth and rapidly reverses the effects of chronic mild unpredictable stressors on the spines (restoring their mature mushroom shape and increasing their numbers), effects that occur within two hours in association with its rapid effects on behaviors that resemble human depression.
About 50–70% of treatment-resistant depressed patients respond to ketamine. However, about one-third of the population has a common genetic variation of BDNF in which one or both valine amino acids that make up the typical val-66-val allele are replaced with methionine (producing val-66-met proBDNF or met-66-met proBDNF). The methionine variations result in the BDNF being transported less easily within the cell. Patients with these poorly functioning alleles of BDNF are less likely to get good antidepressant effects from treatment with ketamine.
Ketamine in Animal Studies
Researcher Pierre Blier reviewed the effects of ketamine on the neurotransmitters serotonin, norepinephrine, and dopamine. In rodents, a swim stress test is used to measure depression-like behavior. Researchers record how quickly the rodents give up trying to get out of water and begin to float instead. Blier found that ketamine’s effects on swim stress were dependent on all three neurotransmitters. For dopamine, ketamine’s effects were dependent on increases in the number of dopamine cells firing, not on the firing rate, and for norepinephrine, ketamine’s effects were dependent on increases in burst firing patterns. Each of these effects was dependent on glutamate activity at AMPA receptors. Given these effects, Blier believes that using ketamine as an adjunct to conventional antidepressants that tend to increase these neurotransmitters may add to its clinical effectiveness.
Important Anecdotal Clinical Notes
Blier reported having given about 300 ketamine infusions to 25 patients, finding that two-thirds of these patients responded, including one-third who recovered completely, while one-third did not respond to the treatment. Patients received an average of 12 infusions, not on a set schedule, but according to when they began to lose response to the last ketamine infusion. If a patient had only a partial response, Blier gave the next ketamine treatment at a faster rate of infusion and was able to achieve a better response. These clinical observations are among the first to show that more than six ketamine infusions may be effective and well tolerated. Read more
Saffron Is An Effective Treatment for Mild Depression
Saffron, the expensive yellow spice derived from the plant Crocus sativus, was the subject of a recent meta-analysis in the journal Human Psychopharmacology. The meta-analysis included six studies of a total of 230 adult outpatients with major depressive disorder. In two of these studies, 30mg/day of saffron extract was as effective as 20mg/day of the antidepressant fluoxetine and 100mg/day imipramine for the treatment of mild to moderate depression had been in other studies.
Saffron is suggested to have anticancer, anti-inflammatory, antioxidant, and antiplatelet effects, and current clinical trials are exploring whether it could prevent and treat Alzheimer’s disease.
The current study was an effort to systematically analyze clinical trials on saffron to establish treatment parameters such as dosage in addition to safety information.