Child Network
A STUDY ASSESSING YOUR CHILD’S MOOD AND BEHAVIOR
Parents, if your child (aged 2–12) has mood or behavioral difficulties, we would like to enlist your participation in a study called the Child Network. Parents who enroll in the study will complete an online rating checklist of your child’s symptoms once a week by a secure web-based system.
In addition, adults who have been diagnosed with depression or bipolar disorder and are the biological parent of a child (ages 2–12) who is currently healthy and has no troublesome mood or behavioral symptoms may also be eligible to participate in this study.
If you are interested in participating in this study, please click here to access informed consent documents. For more information, call 301-530-8245 or email questions to childnetworkbnn@gmail.com.
More Information:
We are reaching out to you to let you know about a new study that the Bipolar Collaborative Network is implementing called the Child Network. Our goal is to foster collective knowledge about childhood bipolar and other disorders by acquiring data about children who have or may be vulnerable to childhood-onset mood and bipolar disorders and associated conditions. Depressive, oppositional, and bipolar disorders as well as anxiety in very young children have not been well studied for their course and for treatment effectiveness and tolerability. Our goal is to compile and analyze the varied treatments for children with these disorders. How well a child responds to a specific treatment may provide new preliminary information of use to others, and assist parents and physicians in assessing treatment options.
The Child Network is specifically for parents of children ages 2 to 12 who have few symptoms, minor (prodromal) symptoms, or the full onset of their illness, including bipolar disorder, prior to age 13. We also seek parents who have been diagnosed with a bipolar disorder to rate their own children who are genetically at higher risk for a mood disorder but as yet display no symptoms. A small percent of these children may eventually develop an anxiety, depressive, or bipolar disorder in childhood. It is estimated from several studies that more than ¼ of adults in the United States with a confirmed diagnosis of bipolar disorder experienced a very early onset in childhood (before age 13). However, many of these individuals did not receive treatment for their illness for 10-15 years following initial onset of the illness, and this delay was associated with a more difficult course of illness.
Participating in the Child Network will primarily involve a weekly ten-minute parental assessment of their child, as well as confidential disclosure of medications and other treatments and any side effects that occur in the prior week. There will also be a short demographics questionnaire and a once a year more detailed symptom checklist. This study does not involve treatment. The network is only meant to document what is currently being done in the community. The child of participating parents will continue to be treated according the child’s physician’s preferences. Studies in this network will not involve randomized or controlled clinical trials or use of placebo, but rather will examine what agents and their combinations that children are already taking are effective, and for which children.
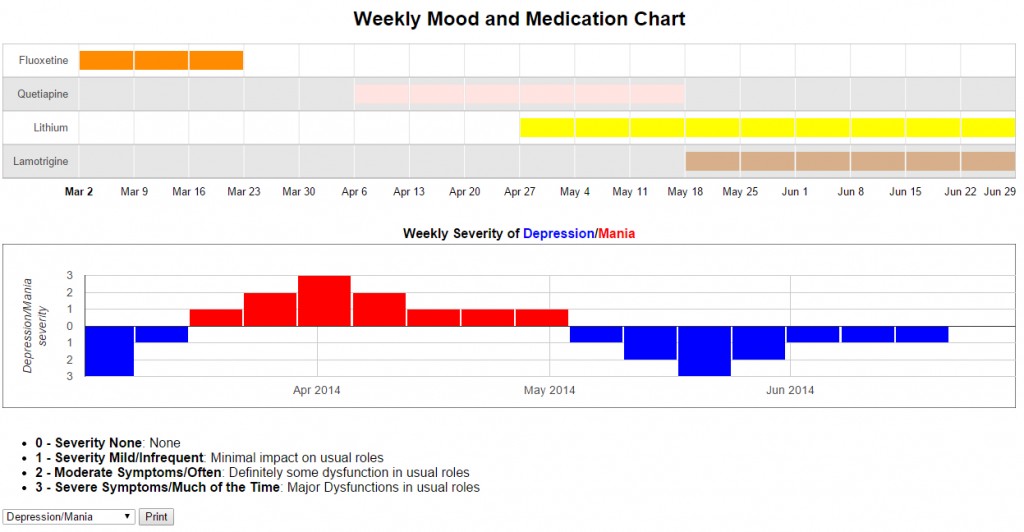
We believe that this network will also benefit its participants. Parents will be able to print out results of the ongoing brief weekly ratings in a graphic form so that the course of the child’s symptoms can easily be visualized. Also, although family members already have access to general information provided in the Bipolar Network News (BNN), participation in this study may help attune parents to the complexities of treatment and engender more careful reading of the BNN and other literature.
We hope that this brief description of the Child Network study helps to orient you to its purpose.
Thank you for your time.
Robert M. Post, MD and Michael Rowe, PhD
Bipolar Collaborative Network , and
Robert L. Findling, MD, MBA, Principal Investigator
This research study is IRB approved by the Johns Hopkins University School of Medicine
Principal Investigator: Robert L. Findling, MD, MBA IRB Study #00026940