“Epigenetic Changes After Trauma May Be Adaptive, Contribute to Resilience”
Originally From Psychiatric News Update
In recent years, research throughout the scientific and medical community has suggested a link between trauma and epigenetic changes, chemical modifications that affect gene activity without actually changing the gene’s DNA sequence. The assumption has been that epigenetic changes in the context of trauma are inherently bad, a form of damage that gets passed from generation to generation. But according to Rachel Yehuda, Ph.D., Endowed Professor of Psychiatry and Neuroscience of Trauma at the Icahn School of Medicine at Mount Sinai, these changes may also be adaptations that promote resilience.
“Sometimes the biological changes in response to trauma or intergenerational trauma are there to help deal with the problem of trauma, not compound its effects,” Yehuda said. “The survival advantage of this form of intergenerational transmission depends in large part on the environment encountered by the offspring themselves.”
Yehuda described this phenomenon as a paradox.
“Parental or ancestral trauma may heighten vulnerability to mental health challenges, but epigenetic adaptations may simultaneously facilitate coping mechanisms,” she said. “Trauma increases susceptibility for psychological distress, but also produces adaptations that help us cope with them.”
Yehuda described research she and her colleagues have conducted to tease out how trauma in parents can affect offspring in the context of the biology of posttraumatic stress disorder (PTSD) in Holocaust survivors and their children. As the research unfolded, Yehuda and colleagues found that survivors’ adult children were more likely to have mood disorders, anxiety disorders, and PTSD than Jewish people whose parents did not directly experience the Holocaust. This was especially true of children of Holocaust survivors who had PTSD. The researchers also found that many children of Holocaust survivors had low levels of the stress hormone cortisol, particularly if their parents had PTSD.
Yehuda and colleagues then conducted a series of studies that looked at the role of glucocorticoid receptors — the proteins to which cortisol must bind to exert its effects — and found evidence that these receptors were more sensitive in people with PTSD.
“In practical terms this means that even though someone with PTSD might have lower circulating levels of cortisol in their blood, their cells might react more strongly to the cortisol that is present,” Yehuda said.
Yehuda said that epigenetics provided further insight on the relationship between hypersensitive glucocorticoid receptors, cortisol, and PTSD. She explained the potential role of methylation, which is a chemical reaction in the body in which a small molecule called a methyl group gets added to DNA or DNA-associated proteins.
“Increased methylation generally impedes RNA transcription, whereas less methylation enhances gene expression,” Yehuda said.
In 2015, Yehuda and colleagues conducted a study involving combat veterans who had PTSD and found lower methylation on an important region on the participants’ glucocorticoid receptor gene. The changes were associated with cortisol and glucocorticoid receptor sensitivity in the study participants, suggesting a potential epigenetic explanation for the association between the trauma of combat and PTSD.
Yehuda said that stress-related epigenetic changes may be reversible. For example, one of the studies conducted by her team revealed that combat veterans with PTSD who benefited from cognitive-behavioral psychotherapy showed treatment-induced changes in the methylation of a gene that regulates glucocorticoid receptor sensitivity. Yehuda said that this finding confirmed that healing is also reflected in epigenetic change.
“That we can transform to meet environmental challenge is a superpower. That is resilience,” Yehuda said.” ?
Yehuda then went on to describe the striking and lasting effects of the psychedelics psilocybin and MDMA in trauma and in helping patients confront their fears in a positive and hopeful fashion. These agents which are given with intensive psychotherapeutic support are not yet FDA approved, but preliminary data suggest that they can have dramatic therapeutic effects in trauma and depression. They can help patients change their attitudes to themselves and the world.
Smoking Pot While Pregnant is a No-No
Mom, Don’t Think Smoking Pot When Pregnant is Harmless for your Child
In a new article in Science, Jasmine Hurd reports on a large sample of mothers who smoked pot while pregnant. Their offspring were more anxious, hyperactive, and aggressive and had higher levels of the stress hormone cortisol in their hair at ages 3-6.
When Superstorm Sandy hit, mothers who were stressed and smoked pot while pregnant had children 31 times more like to have oppositional defiant disorder and 7 times more likely to have an anxiety disorder. Stress may interact negatively with the effects of pot.
In fetuses aborted after being exposed to pot while in utero had decreased dopamine receptors in the their amygdala and n. accumbens, a reward center in brain. In animal studies, pregnant mother rodents who were exposed to THC had offspring more likely to use heroin.
DADS’ BEHAVIOR COUNTS TOO. Dad’s exposure to THC as an adult also led to offspring who preferred opiates. This was based on epigenetic changes passed on in the sperm. To the extent that this also happens in humans, one could ask how much of the current opiate epidemic is based on parental use of marijuana. Mom’s and dad’s smoking pot could make their offspring more vulnerable to opiate addiction.
Participation in Sports May Mitigate Genetic Risk for ADHD in School-Aged Children

At the 2021 meeting of the Society of Biological Psychiatry, researcher Keiko Kunitoki and colleagues reported that participation in sports decreased behavior abnormalities in 9- and 10-year-old children at genetic risk for attention deficit hyperactivity disorder (ADHD). Sports were associated with greater hippocampal volume, which was associated with fewer behavioral abnormalities. Kunitoki and colleagues concluded that “participation in team sports mitigated genomic risk for psychopathology at age 9–10 in part through increased hippocampal volume.”
Editor’s Note: These data are consistent with a program called the Vermont Family-Based Approach developed by researcher James Hudziak, who heads the Vermont Center for Children, Youth and Families at the University of Vermont. The program encourages families to practice different domains of wellness, such as music, mindfulness, exercise, and nutrition, among others. The idea is to support emotional and behavioral health, and to do so intensively in families where children show signs of mood and behavioral difficulties or are at risk for these difficulties.
Hudziak analyzed brain scans of 232 children aged 6 to 18 and reported that “practicing an instrument such as the piano or violin increased working memory, gray matter volume in the brain, and the ability to screen out irrelevant noise. Practicing mindfulness increased white matter volume and reduced anxiety and depression. Exercise also increased brain volume and neuropsychological abilities.”
In 2015, researcher Benjamin I. Goldstein reported that 20 minutes of vigorous exercise on a bike improved cognition and decreased hyperactivity in the medial prefrontal cortex in adolescents with and without bipolar disorder, and researcher Danella M. Hafeman reported that offspring of parents with bipolar disorder who exercised more had lower levels of anxiety.
To summarize, engaging in exercise, team sports, music, and meditation/mindfulness are beneficial for all children, and can be especially helpful for those at risk for depression or bipolar disorder. Children who are already symptomatic should additionally be offered something like family focused therapy (FFT), a multi-faceted approach developed by researcher David Miklowitz, in which families of young people at risk for bipolar disorder take part in therapy, learning together about the illness and practicing strategies for communication and coping.
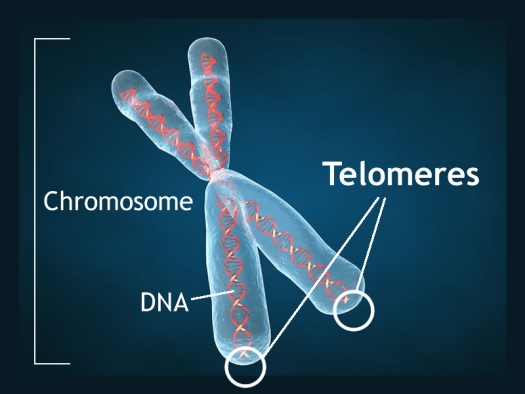
Short Telomeres Associated with Family Risk of Bipolar Disorder
Telomeres are bits of genetic material at the end of each strand of DNA that protect chromosomes as they replicate. Short telomeres have been linked to aging and a variety of medical and psychiatric diseases. Stress and depressive episodes can shorten telomeres, while treatment with lithium can lengthen them.
Telomere length is a heritable trait, and a 2017 study by researcher Timothy R. Powell and colleagues suggests that shorter telomeres are a familial risk factor for bipolar disorder.
The study, published in the journal Neuropsychopharmacology, compared the telomere lengths of 63 people with bipolar disorder, 74 of their immediate relatives (49 of whom had no lifetime psychiatric illness, while the other 25 had a different mood disorder), and 80 unrelated people with no psychiatric illness. The well relatives of the people with bipolar disorder had shorter telomeres than the unrelated healthy volunteers.
Relatives (both well and not) and people with bipolar disorder who were not being treated with lithium both had shorter telomeres than people with bipolar disorder who were being treated with lithium.
Another finding was that longer telomeres were linked to greater volume of the left and right hippocampus, and improved verbal memory on a test of delayed recall. This study provides more evidence that taking lithium increases the volume of the hippocampus and has neuroprotective benefits for people with bipolar disorder.
Link Between Childhood Trauma and Difficult Course of Bipolar Disorder Clarified
A collaboration between Norwegian and French researchers led by Bruno Etain has clarified the pathway by which childhood trauma is linked to worse outcomes among people with bipolar disorder. The researchers, who presented their work in a poster at the 2015 meeting of the Society of Biological Psychiatry, replicated earlier findings by this editor (Robert Post) that patients who experienced trauma as a child had a more adverse course of bipolar disorder. Etain and colleagues found a link between childhood trauma and an earlier age of onset of bipolar disorder, rapid cycling, suicide attempts, and cannabis misuse.
The researchers identified more than 550 patients with bipolar disorder, who answered questionnaires about their history of bipolar disorder and childhood trauma. Their DNA was also analyzed, and the researchers found that the effect of childhood trauma on age of onset was mediated by the presence of common genetic variants in proteins related to stress (the serotonin transporter) and immune function (Toll-like receptors). They also found that the traits of mood lability (or moodiness) and impulsivity mediated the effects of trauma on clinical outcomes.
The lasting epigenetic effects of child maltreatment and adversity noted in the above abstract are consistent with a large literature showing more epigenetic effects in these individuals than in controls. While genetics are important, the impact of the environment is also substantial.
A Note on Genetic Inheritance
Genetic inheritance is not everything, according to J. Craig Venter, pioneering genetic scientist responsible for sequencing the human genome in 2001:
“Human biology is actually far more complicated than we imagine. Everybody talks about the genes that they received from their mother and father, for this trait or the other. But in reality, those genes have very little impact on life outcomes. Our biology is far too complicated for that and deals with hundreds of thousands of independent factors. Genes are absolutely not our fate. They can give us useful information about the increased risk of a disease, but in most cases they will not determine the actual cause of the disease, or the actual incidence of somebody getting it. Most biology will come from the complex interaction of all the proteins and cells working with the environmental factors, not driven directly by the genetic code.”
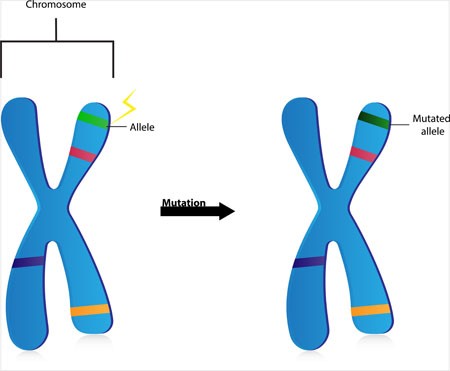
“De Novo” Mutations in Dozens of Genes Cause Autism
Two studies that incorporated data from more than 50 labs worldwide have linked mutations in more than 100 different genes to autism. Scientists have a high level of statistical confidence that mutations in about 60 of those genes are responsible for autism. So-called de novo mutations (Latin for “afresh”) do not appear in the genes of parents without autism, but arise newly in the affected child. The mutations can alter whether the genes get “turned on” or transcribed (or not), leading to disturbances in the brain’s communication networks.
The studies led by Stephan Sanders and Matthew W. State appeared in the journal Nature in late 2014. The identified genes fall into three categories. Some affect the formation and function of synapses, where messages between neurons are relayed. Others affect transcription, the process by which genes instruct cells to produce proteins. Genes in the third category affect chromatin, a sort of packaging for DNA in cells.
Before the new studies, only 11 genes had been linked to autism, and the researchers involved expect to find that hundreds more are related to the illness.
Editor’s Note: This new research explains how autism could be increasing in the general population even as most adults with autism do not have children. It should also put to rest the idea, now totally discredited, that ingredients in childhood immunizations cause autism. It is clearer than ever that kids who will be diagnosed with autism are born with these mutations.
With these genetic findings, the search for new medications to treat this devastating illness should accelerate even faster.
Bottom line: Childhood immunizations don’t cause autism, newly arising mutations in the DNA of parents’ eggs or sperm do. However, parental behavior could put their children and others at risk for the measles and other serious diseases if they do not allow immunizations. The original data linking autism to immunization were fraudulent, and these new data on the genetic origins of autism provides the best hope for future treatments or prevention.
Genetic Variation Predicts Which Type of Antidepressant Will Be Effective
 In a six-month study of Caucasian patients, normal variations in the gene that is responsible for brain-derived neurotrophic factor (BDNF) predicted whether patients would respond better to a selective serotonin reuptake inhibitor (SSRI) antidepressant versus a serotonin and norepinephrine reuptake inhibitor (SNRI) or a tricycle antidepressant. There are several common variants of the BDNF gene, depending on which types of amino acids appear in its coding—valine or methionine. Patients with the most common version, two valines (or Val66Val), responded better to SSRIs. About two-thirds of the population has this version of the gene, which functions most efficiently. The remaining third have at least one methionine in the BDNF gene. Patients with a Met variation responded better to SNRIs and tricyclic antidepressants.
In a six-month study of Caucasian patients, normal variations in the gene that is responsible for brain-derived neurotrophic factor (BDNF) predicted whether patients would respond better to a selective serotonin reuptake inhibitor (SSRI) antidepressant versus a serotonin and norepinephrine reuptake inhibitor (SNRI) or a tricycle antidepressant. There are several common variants of the BDNF gene, depending on which types of amino acids appear in its coding—valine or methionine. Patients with the most common version, two valines (or Val66Val), responded better to SSRIs. About two-thirds of the population has this version of the gene, which functions most efficiently. The remaining third have at least one methionine in the BDNF gene. Patients with a Met variation responded better to SNRIs and tricyclic antidepressants.
The study by R. Colle and colleagues was published in the Journal of Affective Disorders in 2015. Of the patients who were prescribed SSRIs, 68.1% of patients with the Val/Val version responded to the medication after three months, compared to 44% of the patients with a Met version. Of patients prescribed SNRIs or tricyclics, 60.9% of the Met patients reached remission by six months, compared to only 33.3% of the Val/Val patients.
Editor’s Note: In an earlier BNN we reported that according to research published by Gonzalo Laje and colleagues in the journal Biological Psychiatry in 2012, depressed patients with the better functioning Val66Val allele of BDNF respond best to ketamine, while those with the intermediate functioning Val66Met allele respond less well.
ADHD and Bipolar Disorder Are Inherited Separately
While attention-deficit hyperactivity disorder (ADHD) is fairly common among people with bipolar disorder, the genetic risks of inheriting these two illnesses run separately in families. In a recent study of 465 people and 563 of their first-degree relatives by Susan Shur-Fen Gau and colleagues, people with bipolar I disorder were likely to have relatives with bipolar I disorder, and people with ADHD were likely to have relatives with ADHD, but ADHD did not increase risk of bipolar disorder and vice versa.
The researchers hypothesize that other reasons people might develop both disorders include developmental precursors to the illnesses, neurocognitive functioning, sleep problems, and personality traits such as impulsivity and disinhibition.
Editor’s Note: At a recent scientific meeting, Gau and her colleague Kathleen Merikangas said that people with bipolar disorder in the study were five times more likely to have relatives with bipolar disorder. Bipolar disorder and ADHD were comorbid in 37.8% of those with bipolar I disorder, 16.4% in bipolar II disorder, 14% in depression, and 1.1% in normal controls.
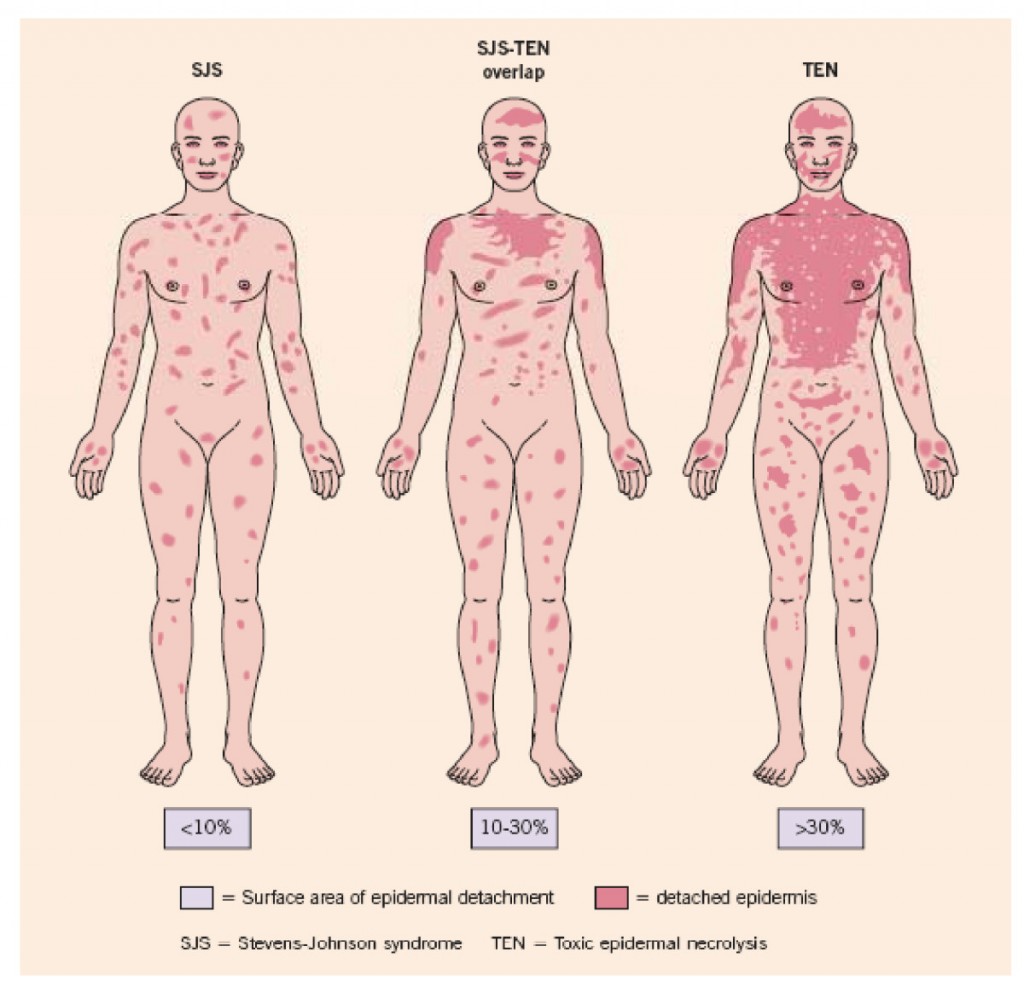
Genetic Test Predicts Risk of Severe Rash While Taking Carbamazepine
Carbamazepine (also known by its trade name Tegretol or, for extended release, Equetro) is one of the most widely used drugs for the treatment of epilepsy, and is relatively underutilized in the treatment of bipolar disorder. One of the reasons is fear of a rare serious rash or other side effects.
The risk of the serious rash ranges from about one in 5,000 to one in 10,000. Loss of white blood cells that fight infection (a condition called agranulocytosis) occurs in about one in 20,000 people taking carbamazepine, while a decrease in white blood cells, red blood cells, and platelets (aplastic anemia) occurs in about one in 100,000 patients.
There is no way of predicting who will develop the blood disorders in reaction to carbamazepine use. A patient should contact their doctor and get a white blood cell count if they develop some warning signs of these conditions, such as a fever or sore throat without other explanation or signs of bleeding or red spots under the skin (called petechiae) that could indicate low platelets.
Genetic Test for Risk of Rash
A genetic test is available that can help estimate the likelihood of the serious rash among certain populations. In those of Asian descent, particularly Han Chinese, Thai, Malaysian, and Indian populations, having a version of the gene HLA-B known as HLA-B*1502 is highly associated with developing the rash. (The odds ratio was 79.84 in a 2013 meta-analysis by Tangamornsuksan et al. in the journal JAMA Dermatology).
In those of northern European or Japanese descent, having a version of the gene HLA-A known as HLA-A*3101 is associated with the severe rash. (Odds ratio for developing the most severe rash was 25.93 in a study of Europeans published by McCormack et al. in the New England Journal of Medicine in 2011 and 10.8 in a study of Japanese published by Ozeki et al. in the journal Human Molecular Genetics in 2011). This HLA-A*3101 gene is present in about 2 to 5% of Europeans and 9% of Japanese.
A mild, non-serious rash with redness and itchiness occurs in about 5 to 10% of patients taking carbamazepine, and almost always goes away quickly upon stopping the drug. For patients taking carbamazepine who develop any rash, stopping the drug is the safest and most conservative thing to do. However, those who have taken the HLA test who know they do not have the risk genes and have only the benign rash might want to consider continuing to take the drug.
Benefits of Carbamazepine
There are a number of reasons why carbamazepine may be worthy of a treatment trial in patients with bipolar disorder who are not doing well on other agents. Carbamazepine works well in many patients with bipolar illness who have some of the common clinical predictors of a poor response to lithium. These include: having dysphoric (anxious, irritable) rather than euphoric mania, having an anxiety or substance disorder comorbidity, having had many prior episodes or rapid cycling (four or more episodes/year), not having distinct episodes with a period of wellness in between, having a sequential pattern of depression followed by mania followed by a well interval (D-M-I rather than M-D-I), having a schizoaffective disorder with delusions or hallucinations that persist after a manic or depressive episode has ended, and having no family history of mood disorders (especially bipolar disorder).
Some patients who do not respond to another anticonvulsant such as valproate do respond to carbamazepine. Patients with bipolar depression who have had a prior history of alcoholism may also do particularly well on carbamazepine. A benefit of the long-acting version of carbamazepine called Equetro is that it can be taken at bedtime and thus help with sleep and minimize daytime side effects.
Editor’s Note: Carbamazepine induces liver enzymes called CYP3A4 that increase the metabolism (breakdown) of carbamazepine and other drugs. Several drugs that inhibit 3A4 (such verapamil and erythromycin) prevent the breakdown of carbamazepine, causing blood levels of the drug to increase and produce side effects. If you are taking carbamazepine, tell your pharmacist so he or she can monitor any other drugs you are taking for potential interactions with carbamazepine.
Knowing about the rare skin and blood side effects of carbamazepine and some of the clinical predictors of a good response to the drug may be helpful in determining whether the potential benefits of carbamazepine outweigh the risks.