Short Telomeres Associated with Family Risk of Bipolar Disorder
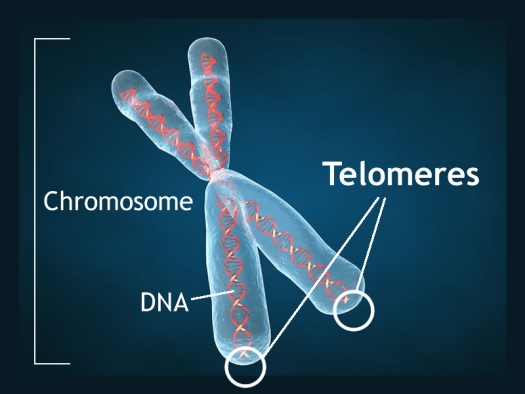
Telomeres are bits of genetic material at the end of each strand of DNA that protect chromosomes as they replicate. Short telomeres have been linked to aging and a variety of medical and psychiatric diseases. Stress and depressive episodes can shorten telomeres, while treatment with lithium can lengthen them.
Telomere length is a heritable trait, and a 2017 study by researcher Timothy R. Powell and colleagues suggests that shorter telomeres are a familial risk factor for bipolar disorder.
The study, published in the journal Neuropsychopharmacology, compared the telomere lengths of 63 people with bipolar disorder, 74 of their immediate relatives (49 of whom had no lifetime psychiatric illness, while the other 25 had a different mood disorder), and 80 unrelated people with no psychiatric illness. The well relatives of the people with bipolar disorder had shorter telomeres than the unrelated healthy volunteers.
Relatives (both well and not) and people with bipolar disorder who were not being treated with lithium both had shorter telomeres than people with bipolar disorder who were being treated with lithium.
Another finding was that longer telomeres were linked to greater volume of the left and right hippocampus, and improved verbal memory on a test of delayed recall. This study provides more evidence that taking lithium increases the volume of the hippocampus and has neuroprotective benefits for people with bipolar disorder.
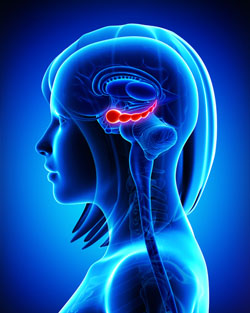
Specific Regions of Hippocampus Linked to Bipolar Disorder
It has been clear for some time that the volume of the hippocampus, a brain region implicated in mood and memory processing, plays a role in bipolar disorder. A 2017 article by researcher Bo Cao and colleagues in the journal Molecular Psychiatry links loss of volume in specific sub-regions of the hippocampus with bipolar disorder.
The study by Cao and colleagues used magnetic resonance imaging (MRI) and a special segmentation technique to compare the volume of certain hippocampal sub-regions across people with bipolar disorder, people with major depression, and healthy control participants.
Participants with bipolar disorder had lower volumes in subfield 4 of the cornu ammonis, two cellular layers (the granule cell layer and the molecular layer), and the tail part of the seahorse-shaped hippocampus compared to the other subjects. Participants with bipolar I disorder had particularly severe volume loss in these areas.
Cao and colleagues also found that volume loss progressed along with the illness. The volumes of the right cornu ammonis, the molecular layer, and the subiculum decreased further in patients who had bipolar disorder for longer. As manic episodes increased, the volume of both sides of the cornu ammonis and the hippocampal tail decreased.
In Rats, Dad’s Cocaine Use Affects Son’s Spatial Memory
Evidence is mounting that certain behaviors by parents can leave marks on their sperm or eggs that are passed on to their offspring in a process called epigenetics. In a recent study by researcher Mathieu Wimmer and colleagues, male rats that were exposed to cocaine for 60 days (the time it takes for sperm to develop fully) had male offspring who showed diminished short- and long-term spatial memory compared to the offspring of male rats that were not exposed to cocaine. Female offspring were not affected in this way.
The spatial tasks the offspring rats completed depended heavily on the hippocampus. Wimmer and colleagues believe that cocaine use in the fathers decreased the amount of a brain chemical called d-serine in the offspring. D-serine plays a role in memory formation and the brain’s ability to form synaptic connections. Injecting the offspring of rats who were exposed to cocaine with d-serine before the spatial memory tasks normalized the rats’ performance.
Agomelatine in an Animal Model of PTSD
At the 2014 meeting of the International College of Neuropsychopharmacology, researcher Joseph Zohar presented a poster on the effects of early post-stressor intervention with the drug agomelatine in animals who showed behavioral and molecular responses to stress that served as a model of post-traumatic stress disorder (PTSD).
Agomelatine is available clinically as an antidepressant in Canada and Europe (but not in the US), and can also reduce anxiety and re-synchronize circadian rhythms. Agomelatine is a melatonin (MT1/MT2) receptor agonist and a serotonin 5HT2C antagonist (increasing dopamine and norepinephrine in the frontal cortex).
Long-term behavioral, molecular and structural effects of the drug were assessed in animals. Adult male Sprague-Dawley rats were exposed to the scent of a predator for 10 minutes, and one hour later they were treated acutely for this stress with agomelatine (50mg/kg i.p.) or placebo.
Agomelatine decreased the prevalence of extreme, PTSD-like behavioral and molecular responses to the stressor, such as freezing in place and increased corticosterone. Agomelatine also normalized decreases in brain-derived neurotrophic factor (BDNF) observed in the dentate gyrus of the hippocampus, the cortex (layer III), and the basolateral amygdala. In line with this, agomelatine-treated stressed animals displayed significantly increased number and length of dendrites at glutamate synapses in the hippocampus (including the dentate gyrus and CA1) and reversed the hippocampal neuronal retraction observed in the rats who were given the placebo.
Agomelatine also affected the expression of clock genes in the rats, which regulate biorhythms. These genes lead to the production of the major clock gene proteins Per1 and Per2. Agomelatine normalized Per1 increases in three parts of the brain: the CA3, another glutamate synapse near the dentate gyrus; the suprachiasmatic nucleus over the optic chiasm, important for circadian rhythms; and the basolateral amygdala. Per2, a protein that also drives circadian rhythms, increased in the CA1 synapse of the hippocampus, the suprachiasmatic nucleus and the basolateral amygdala of the stressed rats.
The researchers concluded that the data provide “initial evidence that a single dose of agomelatine administered in the acute aftermath of stress promotes recovery while promoting enhanced neuronal and synaptic plasticity and connectivity in the secondary prevention of PTSD in this model.”
Anti-Coagulants May Treat Psychosis
Schizophrenia is generally associated with a reduction in volume of the hippocampus. It is thought that the illness results from a predisposition that is triggered by an event such as a trauma or drug use that causes the release of chemicals that damage neurons. Recently, following the finding that five patients with schizophrenia and recurrent deep vein thrombosis (a blood clotting disorder) achieved remission of their psychosis after being treated with the anticoagulant warfarin (Coumadin) and have been able to go years with antipsychotic treatment, researchers Silvia Hoirisch-Clapauch and Antonio Egidio Nardi searched the existing literature for any connection between blood clotting and hippocampal neurogenesis, hoping to find a protein that could encourage neuronal growth in the hippocampus.
They found tissue-plasminogen activator (tPA), which facilitates the conversion of plasminogen to plasmin (the major enzyme responsible for breaking down blood clots) and plays a role in the repair of hippocampal neurons after stress. Low tPA activity is associated with clotting disorders and psychotic events. The drug warfarin reduces blood clotting, and also increases tPA activity, which may explain its effectiveness in treating the five patients who had both schizophrenia and deep vein thrombosis.
Abnormalities in schizophrenia that could be related to low tPA include deficient dopamine transmission at D1 receptors in the prefrontal cortex, impaired cleavage of the precursor to brain-derived neurotrophic factor (pro-BDNF, which can kill cells) to mature BDNF (which helps cells survive), abnormal NMDA receptor-mediated signaling, reduced Akt phosphorylation, and abnormal activation of reelin. The researchers found that all five patients had more than one tPA-related vulnerability to deep vein thrombosis, including high fasting blood insulin, high homocysteine, altered prothrombin activity, and anti-phospho-antibodies.
The same investigators have also examined whether plasmin plays a role in schizophrenia. Read more
Mood-Stabilizing Drugs Increase Growth in Hippocampal Neurons
Lithium is known for protecting neurons by inducing neurotrophic factors and inhibiting cell death factors. In a new study, other mood-stabilizing drugs had similar neuroprotective and neurotrophic effects on cultured neurons from the hippocampus.
At the 2014 meeting of the International Society for Bipolar Disorders, CH Lee et al. presented evidence that lithium, carbamazepine, valproic acid, and lamotrigine all increase the outgrowth of dendrites from these cultured neurons. Therapeutic levels of these drugs increased the production of proteins like brain-derived neurotrophic factor (BDNF), postsynaptic density protein-95 (PSD-95), neurolignin 1 (NLG 1), beta-neurexin, and synaptophysin. However, so far only lithium has been shown to increase the volume of the human hippocampus as measured with MRI.
The Myth of Neurogenesis in Adult Primates Debunked
In a plenary lecture at the Collegium Internationale Neuro-Psychopharmacologicum (CINP) in Istanbul in 2012, Pasco Rakic, professor of neuroanatomy at Yale University, may have debunked a myth of modern medicine, one that we have cited in many previous BNNs. Despite what has been written by famous neuroscientists and published in the most prestigious journals, including Science, Cell, and PNAS, based on data in rodents, Rakic presented evidence that neurogenesis does not occur to any substantial extent in adult primates.
Two decades ago, data in rodents and other species clearly indicated that neurogenesis, the creation of new neurons, occurred in adult animals, especially in the dentate gyrus of the hippocampus. Thousands of papers were written on the subject, and neurogenesis was understood to be possible in adult humans as well. It was even suggested based on data in rodents that the mechanism of action of antidepressants was dependent on neurogenesis. However, Rakic argues that most of the research on neurogenesis was based on faulty data.
Rakic found that while rats do have neurogenesis into adolescence, it diminishes greatly in older adult animals. In primates, neurogenesis in the cortex ends before birth. In the primate dentate gyrus of the hippocampus, there is some postnatal neurogenesis, but it rapidly drops toward zero in the first months of life. Rakic concludes: “We are as old as our neurons…or slightly younger.”
Why should primates have permanent stores of neurons when rodents and other lower animal species get new ones further into their lifespan? Rakic postulates that for primates, neurons must hold experience-dependent memories necessary for the survival of the species, and turning them over would endanger the permanence of this memory. Whatever the reason, it is disappointing to find out that the revolutionary discovery of adult neurogenesis in rodents so widely presumed to also occur in adult primates and humans may not be correct.
This has clinical implications. If we don’t get replacement hippocampal neurons like rats do, it is even more important to protect the billions of neurons that we do have. There are many things that endanger neurons, including inflammation, oxidative stress, high cortisol, poor diet, psychosocial adversity, and episodes of depression and mania. Greater numbers of mood episodes are associated with increasing degrees of cognitive dysfunction because of these many factors. A startling statistic from Denmark by Lars Kessing is that having four or more hospitalizations for depression (either unipolar or bipolar) doubles the risk for a diagnosis of dementia in late life. Thus, it looks like too many episodes hurt the brain.
However, on the positive side, the mood stabilizers (lithium, lamotrigine, valproate, and carbamazepine) and some atypical antipsychotics prevent episodes and increase the neuroprotective factor BDNF, or brain-derived neurotrophic factor, which facilitates synaptogenesis and helps protect neurons. BDNF is produced in selected neurons in the brain and decreases with stress and affective episodes, further endangering neurons. Since many effective treatments both prevent episodes and their associated decreases in BDNF and also directly increase BDNF, they may be having a dual positive benefit. The evidence is best for lithium having neuroprotective effects that can be directly observed in humans. Thus a not unreasonable mantra for patients with recurrent mood disorders is: “Prevent Episodes, Protect the Brain.”