Long COVID ‘Brain Fog’ Confounds Doctors, but New Research Offers Hope
James C. Jackson, PsyD, a licensed psychologist specializing in neuropsychology and rehabilitation, at Vanderbilt University School of Medicine and author of a new book, Clearing the Fog: From Surviving to Thriving With Long COVID ? A Practical Guide, reports in Medscape July, 2023 : “There’s not a lot of imprecision in the term (brain fog) because it might mean different things to different patients,”
Jackson, who began treating [a patient] in February 2023, said that it makes more sense to call brain fog a brain impairment or an acquired brain injury (ABI) because it doesn’t occur gradually. COVID damages the brain and causes injury. For those with long COVID who were previously in the intensive care unit and may have undergone ventilation, hypoxic brain injury may result from the lack of oxygen to the brain.
An April 2022 study published in the journal Nature found strong evidence that SARS-CoV-2 infection may cause brain-related abnormalities, for example, a reduction in gray matter in certain parts of the brain, including the prefrontal cortex, hypothalamus, and amygdala.
Additionally, white matter, which is found deeper in the brain and is responsible for the exchange of information between different parts of the brain, may also be at risk of damage as a result of the virus, according to a November 2022 study published in the journal SN Comprehensive Clinical Medicine.“
Thus, new data suggest that long COVID is associated with inflammation and both reduced volume of several brain structures and decreases in white matter. These data suggest several novel approaches to therapy that require further study. One is low dose lithium which both increases gray matter volume and white matter integrity. Lithium also has some antiviral properties. This could be combined with anti-inflammatories that could be bought over the counter such as N-acetylcysteine (NAC), acetyl-L-carnitine, and celecoxib.
Note of caution. This is only an untested hypothesis and would need to be discussed with one’s physician before any of these options are considered.
LITHIUM’S AMAZING DIVERSITY OF ASSETS
Editor’s Note: Lithium is vastly underutilized. There is wide spread ignorance about its many assets and misconceptions about its few side effects. Here is an update that should be of interest to potential users, family members, and clinicians.
Lithium:
- Prevents unipolar and bipolar depression
- Augments effects of antidepressants in unipolar depression
- Potentiates the effects of atypical antipsychotics in treating mania and depression
- Reduces inflammation
- Normalizes some aspects of cardiovascular risk
- Normalizes secretions for monocytes and leukocytes
- Increases neurogenesis, BCl-2, and hippocampal and thalamic volumes
- The increases in neuroprotective factors occurs at brain levels below typical therapeutic dosages
- Protects against memory deterioration
- Lowers dementia risk in old age
- Reduces suicide clinically and at minute concentrations in the water supply
- Lengthens telomeres and increases longevity
- Reduces size of lesions in models of stroke, AIDS, and Huntington’s chorea
- Normalizes circadian rhythms
- Reduces manic-like behavior induced by clock gene mutations
- Prevents calcium currents and increased firing rate in stem cells from bipolar patients
- Induces minimal to no weight gain on long term follow up
- Does not increase risk of kidney failure when given at blood levels of .6 to .8 blood levels
- Protects against spine and hip osteoporosis
Conclusion: With so many assets and so few liabilities, physicians and patients should reconsider the benefits of lithium and use it more often, not only in the few who respond to it as a monotherapy, but as a adjunct to the many other treatments of bipolar disorder. This should be a “no brainer” as lithium will very likely help some have fewer problems from their illness and may even help them live longer.
Many of these points are summarized in the open access publication: Robert M Post, The New News About Lithium: An Underutilized Treatment in The United States, Neuropsychopharmacology accepted article preview 4 October 2017; several new updates have been added from the International Society on Bipolar Disorders meeting, Chicago, June, 2023.
Lumateperone Normalizes Pathological Levels of Acute Inflammation and Stimulates Important Pathways Involved in Mood Regulation
Highlights from Posters Presented at the Society of Biological Psychiatry Meeting, April 27-29, 2023 in San Diego
Sophie Dutheil of Intra-Cellular Therapies, Inc. reported that “In male and female C57BL/6 mice subjected to an acute stress or immune challenge, lumateperone reduced elevated levels of key proinflammatory cytokines. A number of key genes and pathways associated with the maintenance of tissue integrity and blood-brain barrier function were also altered by a single dose of lumateperone. Furthermore, we found that lumateperone administration conferred anxiolytic- and antianhedonic-like properties while enhancing the mTORC1 signaling pathway in the PFC.”
Inflammatory marker CRP predicts worse course of adolescent bipolar disorder
Sudhir Karthikeyan in Ben Goldstein’s lab in Toronto reported in Brain Behav Immun (2022) that in 79 adolescents the inflammatory marker CRP (C-Reactive Protein) was higher and the anti-inflammatory cytokine Il-10 was lower during the most ill periods compared to normal volunteers. “Moreover, higher CRP levels (p = 0.009) at intake predicted greater time to recovery from the index symptomatic episode.” They concluded that: “In the first repeated-measures study on this topic in adolescents with BD, we found evidence that CRP, an inexpensive and ubiquitous blood test, may be useful in predicting the prospective course of BD symptoms. “
Hyperinsulinemia Associated Depression
Haider Sarwar writes in Clinical Medicine Insights (2022) that “Hyperinsulinemia promotes fat accumulation, causing obesity. Being an inflammatory state, obesity can induce further inflammation and is a risk factor for HPA (hypothalamic pituitary axis) dysregulation through hypercortisolism-related hyperglycemia….A disruption on SNS (sympathetic nervous system) activity increases insulin levels, and induces glycogenolysis in the liver and lipolysis in adipose tissue during hypoglycemia. Hyperglycemia-hyperinsulinemia exacerbates inflammation and increases the oxidative stress along with regulating the levels of norepinephrine in the brain sympathetic system. Increased inflammatory cytokines have also been shown to disrupt neurotransmitter metabolism and synaptic plasticity which play a role in the development of depression via inhibiting serotonin, dopamine, melatonin, and glutamate signaling. An increased level of plasma insulin over time in the absence of exercising causes …an increase in insulin resistance due to obesity and further culminates into depression….. Triple therapy with SSRI, bupropion, and cognitive behavioral therapy aids in improving glycemic control, lowering fasting blood glucose, decreasing the chances of relapse, as well as decreasing cortisol levels to improve cognition and the underlying depression.”
Translocator Protein Levels in Brain Predict Response to Anti-Inflammatory Celecoxib in Major Depressive Disorder
Gliosis describes changes in glia that result from damage to the central nervous system. Researchers can use PET scans (positron emission tomography) to measure the extent of gliosis in the brain. But a new study explored whether these PET scans could instead be used to determine who might respond to a given medication.
Researcher Sophia Attwells and colleagues reported in the journal Biological Psychiatry in 2020 that people with high levels of translocator protein (TSPO), a measure of gliosis and inflammation, had a better antidepressant response to the anti-inflammatory drug celecoxib than patients who started out with lower levels of TSPO.
The study participants, who had treatment-resistant depression, all received 200mg of the anti-inflammatory drug celecoxib twice/day for eight weeks on an open (non-blind) basis. Before they began taking celecoxib, the participants received PET scans to measure translocator protein total distribution volume (TSPO VT) in the prefrontal cortex and the anterior cingulate cortex.
Patients with high levels of TSPO showed greater reductions in depression ratings over the course of the study than those with normal levels of TSPO at baseline.
Attwells and colleagues conclude that “this personalized medicine approach of matching a marker of gliosis to [an anti-inflammatory treatment] …should be applied in early development of novel therapeutics, in particular for [treatment-resistant depression].”
Editor’s Note: These findings are of considerable importance, as they are among the first to indicate that measures of inflammation may predict response to an anti-inflammatory medication such as celecoxib. In a 2013 article in the journal JAMA Psychiatry, Charles L. Raison and colleagues reported that patients with high levels of the peripheral inflammatory marker CRP saw marked improvement in their depression when they received the anti-inflammatory treatment infliximab while those with lower or normal levels of inflammation actually worsened.
Inflammation Predicts Lower Frontal and Temporal White Matter Volumes in Early-Stage Bipolar Disorder
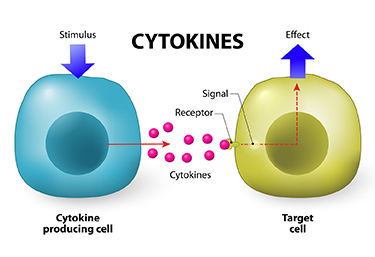 At the 2019 meeting of the International Society for Bipolar Disorders, researcher David Bond found that seven inflammatory cytokines predicted lower white matter volumes in the left frontal and bilateral temporal lobes, as well as in the cingulate and inferior frontal gyri. Cytokines are secreted by some immune cells and send signals that can produce an effect in other cells.
At the 2019 meeting of the International Society for Bipolar Disorders, researcher David Bond found that seven inflammatory cytokines predicted lower white matter volumes in the left frontal and bilateral temporal lobes, as well as in the cingulate and inferior frontal gyri. Cytokines are secreted by some immune cells and send signals that can produce an effect in other cells.
Bond noted that greater inflammation did not predict lower parietal or occipital white matter volumes, suggesting that inflammation had a greater effect on white matter volume in those parts of the brain most closely linked to mood disorders.
Inflammation Associated with Cognitive Deficits
At the 2019 meeting of the International Society for Bipolar Disorders, researcher Katherine E. Burdick and colleagues at Brigham and Women’s Hospital and Harvard Medical School reported that in 240 patients with bipolar disorder who were not currently having a manic or depressive episode, markers of inflammation were associated with cognitive deficits.
Inflammation was associated with cognitive deficits in general, and there were also some relationships between specific inflammatory markers and types of cognitive processing. They found that the inflammatory markers TNF-alpha, TNFR1, and TNFR2 influenced cognitive flexibility. The inflammatory marker VEGF influenced reward processing, while IL-6/IL-6r influenced spatial processing. IL-1beta and IL-1RA influenced social cognition.
Burdick and colleagues found it was important to include both primary and secondary mediators of inflammation in their research “as the effects of the primary pro-inflammatory cytokines can be blocked by a number of decoy receptors and soluble antagonists.” Elevations in these can provide additional information about the function of the immune system.
Editor’s Note: Targeting inflammation with the anti-inflammatory treatments minocycline and celecoxib has been shown to improve depression. Now the role of anti-inflammatory drugs in improving cognition deserves further attention.
Infliximab Helps the Subgroup of Bipolar Depressed Patients Who Faced Adversity in Childhood
At the 2019 meeting of the International Society for Bipolar Disorders, researcher Mike Cosgrove and colleagues described a study of the immune-suppressing drug infliximab in adults with bipolar disorder. The researchers found persistent significant improvements on infliximab only in those with bipolar disorder who also had a history of childhood adversity. Childhood adversity is consistently associated with elevated levels of inflammatory cytokines, and baseline inflammation may be a prerequisite for a positive effect from infliximab, which works by blocking the inflammatory cytokine TNF alpha.
Links Between Mixed Depression, Insulin Resistance, Inflammation, and Cognitive Deficits
At the 2019 meeting of the International Society for Bipolar Disorders, researcher Roger McIntyre discussed links between obesity, diabetes, and cardiovascular problems; increased inflammation; and decreased functioning of the neural networks involved in cognition.
He and his colleagues analyzed 121 studies that included empirical research and meta-analyses. McIntyre and colleagues found that patients with higher levels of inflammatory markers have more insulin resistance and cognitive dysfunction. A meta-analysis revealed that the inflammatory markers IL-6, TNF alpha, and CRP were significantly elevated in people with bipolar disorder compared to normal controls, while IL-1B was not.
People with depression who had a few manic traits (mixed depression) were particularly likely to have insulin resistance and elevated levels of pro-inflammatory markers.
People with mixed depression have increases in inflammation and increased incidence of cardiovascular disorder. People experiencing a first episode of mixed depression who are overweight show increased signs of brain aging.
In studies McIntyre and colleagues analyzed, diabetes or pre-diabetes occurred in 50% of depressed patients, and these patients had the greatest amount of cognitive dysfunction.
Treatment
McIntyre noted that taking the antipsychotic drug lurasidone for bipolar depression worked best in both adults and children who had elevated levels of CRP at baseline. The fast-acting antidepressant ketamine also works well in those who show baseline inflammation .
The anti-diabetes drug liraglutide (Victoza, Saxenda) improves mixed depression symptoms and cognition in obesity, diabetes, and mixed depression. Liraglutide belongs to a class of drugs called glucagon-like peptide-1 (GLP-1) receptor agonists or incretin mimetics. They work by increasing insulin release from the pancreas and decreasing excessive glucagon release.
McIntyre now routinely uses liraglutide for cognitive deficits in patients with obesity or diabetes, including patients with mixed depression. It is injected under the skin at 0.6 mg daily, then the dosage is increased to 1.2 mg and then 1.8 mg. Victoza reduces major cardiovascular events in those with type 2 diabetes. The higher-dose Saxenda (3mg) can be used for weight control.
Another anti-diabetes drug, pioglitazine, has also been reported to be helpful in bipolar depression.
McIntyre found that the antibody infliximab, which can be used as an intravenous treatment for chronic inflammation and works by blocking the effects of TNF-alpha, did not improve depression, but did improve cognition.
McIntyre also supports the use of acetyl-L-carnitine, a potential adjunctive treatment that can reverse the insulin resistance that often occurs with obesity and thus could theoretically improve cognition.
McIntyre described preliminary literature suggesting the effectiveness of drugs such as statins, calcium channel blockers, and biguanides such as the diabetes treatment metformin in reducing inflammation.
Bariatric surgery to reduce the size of the stomach was another option discussed by McIntyre. He said the intervention is safe for patients with bipolar disorder and can help them recover cognitive function.
McIntyre noted that offspring of a mother with obesity have decreased response to sensory cues, reward preference, cognitive control, and motor control. Obesity and the inflammation that goes along with it apparently affect offspring via epigenetic mechanisms, meaning obesity may change the structure of inherited DNA (without changing its sequence).




