TDCS Promising for a Range of Illnesses
 Transcranial direct current stimulation (tDCS) shows promise for a range of problems. In new research presented at the 2014 meeting of the Society of Biological Psychiatry, it was reported to be effective for improving cognition in bipolar disorder, alleviating depression, and reducing hallucinations.
Transcranial direct current stimulation (tDCS) shows promise for a range of problems. In new research presented at the 2014 meeting of the Society of Biological Psychiatry, it was reported to be effective for improving cognition in bipolar disorder, alleviating depression, and reducing hallucinations.
How TDCS Works
At the meeting, researcher Marom Bikson discussed tDCS technology. The treatment can be delivered with a 12-volt battery. The anode directs current inward and is excitatory, while the cathode directs current outward and is inhibitory. The dendrites at the top of neurons under the anode are hyperpolarized by the tDCS, leading to relative depolarization of the cell soma, thus increasing excitation. TDCS, unlike repetitive transcranial magnetic stimulation (rTMS), which causes cells to fire, is only neuromodulatory, inducing minor changes in membrane polarization.
TDCS Improved Cognition in Bipolar Disorder
At the 2014 meeting of the American Psychiatric Association, Roberto Delle Chiaie et al. reported that two mA tDCS for 20 minutes for 15 days (anode over the left prefrontal cortex and cathode over the right cerebellum) improved immediate and delayed recall, trail making with a pointer, and motor coordination in 17 euthymic bipolar patients. This very promising result deserves further study and replication.
Antidepressant Effects of TDCS
At the 2014 meeting of the Society of Biological Psychiatry, Collen Loo reported that tDCS had positive effects in depressed patients compared to sham treatment. This complements a 2013 article by Brunoni et al. in JAMA Psychiatry that tDCS plus the selective serotonin reuptake inhibitor (SSRI) antidepressant sertraline (Zoloft) was more effective than either treatment alone.
TDCS for Treatment-Resistant Hallucinations
Jerome Brunelin et al. reported at the meeting that tDCS had positive effects in patients with schizophrenia who had hallucinations that resisted treatment. The positive electrode (anode) was placed over the left prefrontal cortex and the negative electrode (cathode) over the left temperoparietal area, where hallucinations are thought to originate. Stimulation was at two mA for 20 minutes, five days per week for two weeks. Effects lasted as long as 30 days and were associated with reduced functional connectivity of these brain regions.
Low frequency (1Hz) rTMS, which decreases neural activity, also improves refractory hallucinations when applied over the temperoparietal area, which is important for language. Placing the cathode over this area in tDCS is also inhibitory, so comparisons of rTMS with tDCS for suppressing hallucinations would be of great interest and importance.
Transcranial Magnetic Stimulation Continues To Show Effectiveness In Depression
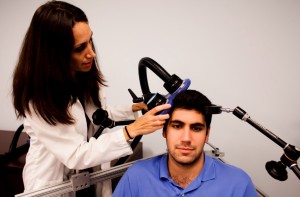 At the 2014 meeting of the Society of Biological Psychiatry, David G. Brock et al. reported that 41 of 67 depressed patients achieved remission (61.2%) after acute treatment with Transcranial Magnetic Stimulation without other medication. After three months of continuation treatment in which patients either received one maintenance TMS session per month or were simply observed, 10 of the 16 receiving active TMS continuation (62.5%) did not relapse, while 7 of the 16 who were only observed (43.8%) did not relapse. While this was not a statistically significant difference, it suggests that continuation TMS should be studied further.
At the 2014 meeting of the Society of Biological Psychiatry, David G. Brock et al. reported that 41 of 67 depressed patients achieved remission (61.2%) after acute treatment with Transcranial Magnetic Stimulation without other medication. After three months of continuation treatment in which patients either received one maintenance TMS session per month or were simply observed, 10 of the 16 receiving active TMS continuation (62.5%) did not relapse, while 7 of the 16 who were only observed (43.8%) did not relapse. While this was not a statistically significant difference, it suggests that continuation TMS should be studied further.
Andrew Leuchter et al. reported that synchronized transcranial magnetic stimulation (sTMS) at a patient’s individual alpha frequency (IAF) was more effective than sham treatment in those with prior treatment resistance (34.2% vs 8.3%) but not different from sham treatment in depressed patients who had never received treatment.
Editor’s Note: This would be important if replicated, as patients with high levels of treatment resistance do not tend to respond well to regular rTMS given at 10Hz and not matched to a patient’s alpha frequency.
RTMS Reduced Smoking
Dinur-Klein Limor reported that 10 Hz (but not 1 Hz) repetitive transcranial magnetic stimulation (rTMS) over the left pre-frontal cortex decreased cigarette consumption when given in combination with a smoking cue.
Lithium Superior to Valproate at Preventing Manias and Depressions
 In a special symposium on bipolar disorder at the 2014 meeting of the American Psychiatric Association, researcher Mike Bauer reviewed a new meta-analysis that showed lithium not only has significant effects in preventing manias, but also depressions. Researcher Geddes et al. had, in a previous study called BALANCE, found that lithium was superior to valproate (Depakote). Together these findings led Bauer to the conclusion that lithium is under-used in the treatment of bipolar disorder, especially in the US, where lithium is prescribed less often than valproate.
In a special symposium on bipolar disorder at the 2014 meeting of the American Psychiatric Association, researcher Mike Bauer reviewed a new meta-analysis that showed lithium not only has significant effects in preventing manias, but also depressions. Researcher Geddes et al. had, in a previous study called BALANCE, found that lithium was superior to valproate (Depakote). Together these findings led Bauer to the conclusion that lithium is under-used in the treatment of bipolar disorder, especially in the US, where lithium is prescribed less often than valproate.
An article by researcher Kessing in the British Journal of Psychiatry in 2012 relied on naturalistic follow up data and also showed that lithium was superior to valproate in preventing hospitalizations.
A study by researcher Willem Nolen indicated that in mono-therapy, levels of lithium in the blood needed to be 0.6 meq/L or higher in order for lithium to work better than placebo. Lithium augmentation that produced lower blood levels of 0.3 meq/L was not significant on its main outcome measure of preventing new episodes. However, compared to treatment as usual, those randomized to lithium used lower doses of atypical antipsychotics, and other data indicated that these patients had fewer suicide attempts and increased hippocampal volume.
Bauer noted that lithium-related goiter and low thyroid are easily treated, and that kidney damage while taking lithium can be prevented by avoiding episodes of lithium intoxication. It is easy to conclude that lithium should be used more often, especially given its positive effects against suicide and brain gray matter and hippocampal volume loss.
N-acetylcysteine Decreases Smoking
It appears that the nutritional supplement n-acetylcysteine (NAC) may be useful for people who want to quit smoking. Researcher Eduardo S. T. Prado et al. reported that compared to placebo, NAC decreased the number of cigarettes a patient smoked per day and the amount of carbon monoxide they exhaled. Participants in the study took 1,500mg of NAC twice a day.
Editor’s Note: It looks as though NAC is effective in most addictions, including gambling, cocaine, heroin, marijuana, alcohol, and now smoking. Since it also helps depressed mood and anxiety in patients with bipolar illness (a finding first reported by researcher Michael Berk et al. in 2008), and can improve trichotillomania and obsessive compulsive disorder (OCD), it could be an important adjunctive treatment for many patients with bipolar illness who also suffer from many of these comorbidities. The usual dose in most of these studies was 500mg twice a day for one week, then 1,000mg twice a day thereafter, as opposed to the doses of 1,500mg twice a day that were used in the smoking study.
Antidepressant Vilazodone Superior to Placebo, Plus No Sexual Side Effects
Vilazodone (Viibryd) was approved by the Federal Drug Administration (FDA) as an antidepressant in 2011. The drug is a serotonin 5-HT reuptake inhibitor and a partial agonist of the serotonin 5-HT1A receptor like the anti-anxiety drug buspirone (Buspar). Neither buspirone nor vilazodone is associated with significant sexual dysfunction, unlike most of the antidepressants that only inhibit the serotonin transporter (selective serotonin reuptake inhibitors or SSRIs). Researcher Leslie Citrome et al. reported at the 2014 meeting of the American Psychiatric Association that at 40mg/day, the rate of remission was 32% on vilazodone versus 20% on placebo.
At the same meeting, researcher Carl Gommoll et al. reported on vilazodone’s side effects. The drug was generally well-tolerated. Side effects that occurred in 5% or more of the patients taking vilazodone and half as many taking placebo included diarrhea, nausea, vomiting, and insomnia.
New Antidepressant Vortioxetine May Improve Cognition and Treatment-Resistant Depression
Vortioxetine (Brintellix) is a new antidepressant that has a range of effects on serotonin receptors, making it different from selective serotonin reuptake inhibitors (SSRIs), the most common type of antidepressants, which work only on the serotonin transporter. Researcher Johan Areberg et al. reported at the 2014 meeting of the American Psychiatric Association that the drug is an antagonist at receptors 5-HT3, 5-HT7, and 5-HT1D; a partial agonist at 5-HT1B; a full agonist at 5-HT1A; and an inhibitor of the 5-HT transporter. The researchers suggested that at doses of 5mg/day, vortioxetine occupies the 5-HT3 receptors and 50% of the serotonin transporter. As dosage increases to 20mg/day, vortioxetine is believed to occupy all of the serotonin targets at clinically relevant levels. Doses of 20mg/day were found to be effective in nine studies. Researcher Gennady Smagin et al. also reported that vortioxetine activates central histamine receptors.
Vortioxetine appears to be useful in patients who have previously failed to respond to antidepressants. Researcher George I. Papakostas et al. reported that in a cohort of about 500 patients who responded inadequately to previous prescriptions of selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs), the 252 taking vortioxetine improved more than the 241 taking the antidepressant agomelatine.
Editor’s Note: Vortioxetine’s superior effects are impressive, as agomelatine, which is approved for use in at least 41 countries including the UK, Canada, and Australia, but is not available in the US, has previously been shown to be more effective than a number of SSRIs in head-to-head comparisons. Agomelatine improves sleep and circadian rhythms via its dual effects as an agonist at melatonin M1 and M2 receptors and an inhibitor of 5HT2C receptors, which results in the release of norepinephrine and dopamine in the frontal cortex.
Vortioxetine may be unique among antidepressants in that it appears to improve cognition. Researcher John E. Harrison et al. reported that patients saw increases in executive function, attention, speed of processing, and memory while taking vortioxetine. This is consistent with studies in aged mice, whose cognition improves more on vortioxetine than on the SSRI fluoxetine, according to researcher Yan Li and colleagues.
New Drug Cariprazine Has Anti-Manic Effects
Cariprazine is a new antipsychotic drug from Hungarian company Gedeon Richter. It functions as a dopamine D3 and D2 partial agonist. The drug has shown significant antimanic effects in three placebo-controlled studies. At the 2014 meeting of the American Psychiatric Association, researcher Robert E. Litman presented findings that 32% of patients with moderate to severe mania improved to a point of minimal or no illness while taking cariprazine, versus 22% who improved similarly while taking placebo. Doses in the studies Litman presented ranged from 3mg/day to 12mg/day.
At the same meeting, researcher Lakshmi N. Yatham discussed cariprazine tolerability. At a mean dose of 7.44mg/day, side effects of cariprazine compared to placebo included akathisia (restless legs) in 20% of patients compared to 5%, extrapyramidal side effects (irregularities in movement) in 13% of patients compared to 5%, vomiting in 9% of patients compared to 4%, and restlessness in 6% of patients compared to 2%. Twelve percent of patients discontinued treatment due to side effects while taking cariprazine, compared to 7% taking placebo. Weight increased by an average of 0.54kg among patients taking cariprazine compared to an average of 0.17kg among those taking placebo. Yatham and colleagues concluded that cariprazine treatment is generally safe and well-tolerated.
It is expected that data on the positive effects of cariprazine in bipolar depression in two placebo-controlled studies will soon be published.
Also at the meeting, researcher Nika Adham et al. reported that in animal studies, cariprazine had greater affinity for the dopamine D3 receptor than aripiprazole (Abilify), another partial agonist at D2 and D3 receptors. D3 receptors are important for the regulation of cognition and mood. It is expected that cariprazine might eventually be useful in the treatment of schizophrenia.
Acetly-l-carnitine May Be Effective in Treatment-Resistant Depression
 Not all patients with unipolar depression respond to the currently available antidepressants. Acetyl-l-carnitine is a compound that enhances mitochondrial function and neuroplasticity and is effective in the treatment of peripheral neuropathy (damage to the peripheral nerves, which sometimes occurs in chemotherapy or diabetes). It is now being investigated as an antidepressant for patients who have not responded to typical antidepressants.
Not all patients with unipolar depression respond to the currently available antidepressants. Acetyl-l-carnitine is a compound that enhances mitochondrial function and neuroplasticity and is effective in the treatment of peripheral neuropathy (damage to the peripheral nerves, which sometimes occurs in chemotherapy or diabetes). It is now being investigated as an antidepressant for patients who have not responded to typical antidepressants.
According to a review of the treatment by S.M. Wang et al. published in the Journal of Psychiatric Research in 2014, acetyl-l-carnitine treated depression better than placebo did in four randomized clinical studies. It was better than placebo and equally as effective as the antidepressant fluoxetine and the atypical antipsychotic amisulpride in various studies of dysthymic disorder. It also improved depressive symptoms in people with fibromyalgia and minimal hepatic encephalopathy (liver damage). The usual dose of acetyl-l-carnitine is 1 to 2 grams/day.
Editor’s Note: The role acetyl-l-carnitine will play in treating people with treatment-resistant unipolar or bipolar depression remains to be better clarified.
Inflammation Predicts Depression Persistence
Links between inflammation and depression continue to be identified in new research. Researcher N. Vogelzangs et al. reported in a 2014 article in Neuropharmacology that inflammatory and metabolic dysregulation in antidepressant users predicted an outcome of depression two years later. Elevated levels of the marker of inflammation Il-6, low HDL (or “good”) cholesterol, high triglycerides, and high blood sugar were associated with poor response to medication and chronicity of depression. Of 315 people treated with antidepressants (average age 43), 138 were in remission at 2 years, while 177 (56.2%) were still depressed. People with four or more types of inflammatory or metabolic dysregulations had a 90% chance of still being depressed at 2 years.
Among inflammatory markers including CRP and TNF-alpha, IL-6 alone was associated with chronic depression. Il-6 can cross the blood-brain barrier. We have previously reported that researcher Scott Russo found that in rats in a depression-like state known as defeat stress (brought about by repeated defeat by a larger rodent), blocking Il-6 can prevent depressive behaviors such as social avoidance or loss of preference for sucrose.
Like inflammation, metabolic abnormalities also complicate depression. Lipid dysregulation and hyperglycemia are associated not only with depression persistence, but also with the new onset of depression in humans.
Vogelzangs et al. conclude that these data “ suggest that inflammatory and metabolic dysregulation worsens depression course owing to reduced [antidepressant] response and that alternative intervention treatments may be needed for depressed persons with inflammatory and metabolic dysregulation.”
It is noteworthy that a 2014 meta-analysis of the anti-inflammatory drug celecoxib (Celebrex) published by Farhad Faridhosseini et al. in Human Psychopharmacology, showed that the drug, often prescribed for arthritis, is effective for unipolar depression when added to patients’ regular treatment.
It remains to be ascertained whether celecoxib’s effects are seen in depression in general, or if they pertain only to the 30% of depressed patients who show inflammation at baseline. Typical markers of inflammation include Il-6, CRP, TNFa, and Il-1.
Statins, prescribed to lower cholesterol, also have anti-inflammatory effects, and are also effective in preventing depression.
Determining treatment approaches for those patients showing signs of inflammation or metabolic irregularities remains a high priority for study. The preliminary data noted here suggest that treating these dysregulations in those with depression may be useful.
Psychotherapy More Effective Than Collaborative Care in Bipolar Depression with Anxiety Disorder Comorbidity
The Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD), a long-term study of treatments for bipolar disorder, recently found that psychotherapy was more effective than their normal collaborative care model (consisting of regular illness evaluation and treatment) for patients with bipolar disorder and a current or lifetime presence of an anxiety disorder.
An anxiety disorder comorbidity is consistently associated with a poor outcome in patients with bipolar disorder. In a 2014 article by Deckersbach et al. in the American Journal of Psychiatry, the STEP-BD research group reported that the effect of psychotherapy was particularly strong in those with comorbid post-traumatic stress disorder (PTSD) or generalized anxiety disorder.
While antidepressants are typically used to treat anxiety disorders in unipolar depression, this has not been proven effective in bipolar disorder. Not only do patients with bipolar disorder tend to respond poorly to antidepressants, but in research collected by this editor Robert Post and colleagues in the Bipolar Collaborative Network, patients with bipolar disorder who had an anxiety disorder fared even more poorly on antidepressants as adjuncts to mood stabilizers than those with bipolar disorder without an accompanying anxiety disorder.
The poor response to antidepressants in bipolar depression in general, and particularly in those with a comorbid anxiety disorder, together with the finding that psychotherapy is highly effective, suggest that adjunctive psychotherapy is a more appropriate choice for patients with bipolar depression and a comorbid anxiety disorder.
The choice of the best pharmacological treatment of this comorbid anxiety disorder deserves specific comparative study. Candidates would include the mood stabilizing anticonvulsants valproate, lamotrigine, and carbamazepine; the atypical antipsychotics with efficacy in bipolar depression (quetiapine, lurasidone, and olanzapine combined with fluoxetine); and those used as an adjunct in unipolar depression (quetiapine again and aripiprazole).