Saffron Is An Effective Treatment for Mild Depression
Saffron, the expensive yellow spice derived from the plant Crocus sativus, was the subject of a recent meta-analysis in the journal Human Psychopharmacology. The meta-analysis included six studies of a total of 230 adult outpatients with major depressive disorder. In two of these studies, 30mg/day of saffron extract was as effective as 20mg/day of the antidepressant fluoxetine and 100mg/day imipramine for the treatment of mild to moderate depression had been in other studies.
Saffron is suggested to have anticancer, anti-inflammatory, antioxidant, and antiplatelet effects, and current clinical trials are exploring whether it could prevent and treat Alzheimer’s disease.
The current study was an effort to systematically analyze clinical trials on saffron to establish treatment parameters such as dosage in addition to safety information.
Study Finds No Substantial Risk of Infant Cardiac Problems from Antidepressant Use During Pregnancy
In the past there has been some concern that selective serotonin reuptake inhibitor (SSRI) antidepressants taken during pregnancy could increase an infant’s risk of cardiac problems. There was particular concern that the SSRI paroxetine could lead to right ventricular outflow tract obstruction, and sertraline could lead to ventricular septal defects. A 2014 study by KF Huybrechts et al. in the New England Journal of Medicine analyzed data from 949,504 women in a Medicaid system from three months before pregnancy until one month after delivery during the years 2000-2007.
Infants born to mothers who had taken antidepressants during their first trimester were compared to infants whose mothers had not taken antidepressants. In total, 6.8% or 64,389 women had used antidepressants in their first trimester.
While the rate of cardiac defects in newborns was greater among those mothers who had taken antidepressants (90.1 infants per 10,000 infants who had been exposed to antidepressants versus 72.3 infants per 10,000 infants who had not been exposed to antidepressants), this relationship diminished as confounding variables were removed. The relative risk of any cardiac defect after taking SSRIs was 1.25, but this decreased to 1.12 when restricted to only those mothers who were diagnosed with depression, and to 1.06 when the researchers controlled for things like depression severity. (All relative risk numbers were calculated with a 95% confidence interval.)
The researchers concluded that there is no substantial risk of increased cardiac defects in children born to mothers who took antidepressants during their first trimester.
The Good and Bad News About Deep Brain Stimulation for Treatment-Resistant Depression
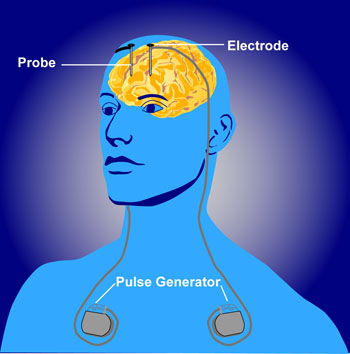 Deep brain stimulation is a treatment in which electrodes are implanted in the brain to treat movement or affective disorders. At the 2014 meeting of the International College of Neuropsychopharmacology, Thomas Schlaepfer reviewed the current status of studies of deep brain stimulation for depression. The bad news is that two double-blind randomized controlled studies are no longer recruiting patients because interim analysis failed to show a benefit to the deep brain stimulation over a sham stimulation. The studies targeted two of the most promising parts of the brain for deep brain stimulation—the subgenual anterior cingulate (important for motivation) and the anterior limb of the internal capsule (which contains nerve fibers going to and from the cerebral cortex), so their failure is a disappointment. However, Helen Mayberg, one of the lead researchers studying the subgenual anterior cingulate, will continue to study this target for deep brain stimulation.
Deep brain stimulation is a treatment in which electrodes are implanted in the brain to treat movement or affective disorders. At the 2014 meeting of the International College of Neuropsychopharmacology, Thomas Schlaepfer reviewed the current status of studies of deep brain stimulation for depression. The bad news is that two double-blind randomized controlled studies are no longer recruiting patients because interim analysis failed to show a benefit to the deep brain stimulation over a sham stimulation. The studies targeted two of the most promising parts of the brain for deep brain stimulation—the subgenual anterior cingulate (important for motivation) and the anterior limb of the internal capsule (which contains nerve fibers going to and from the cerebral cortex), so their failure is a disappointment. However, Helen Mayberg, one of the lead researchers studying the subgenual anterior cingulate, will continue to study this target for deep brain stimulation.
The better news is that Schlaepfer repositioned the electrodes to target a site in the medial forebrain bundle nearer to the ventral tegmental area. After this shift he observed rapid onset of antidepressant response (within two days) in seven of the first eight patients studied, and these responses persisted over many months of follow up. This response was achieved at 2.8 microamps, a lower stimulation current than was used in other studies of deep brain stimulation.
Editor’s Note: Since patients started to feel better when they were still on the operating table, this may offer an opportunity to more rapidly assess effectiveness, do a double-blind study, and see if the findings can be replicated as another mode of achieving rapid-acting and long-lasting antidepressant effects in treatment-resistant patients. Intravenous ketamine has rapid-onset antidepressant effects, but its effects are short-lived.
Antidepressants and Ketamine Reverse Animal Models of Anhedonia and Learned Helplessness
Researcher Tony Pitts presented a study at the 2014 meeting of the International College of Neuropsychopharmacology (CINP) that described the neurobiology of an animal model of depression in rodents. In animal models, researchers provoke depression-like symptoms in animals with the hopes of finding neurobiological clues to human depression. Pitts’ studies explored the effects of acute stressors as well as more chronic long-term stressors such as learned helplessness.
In the rodents, acute stressors caused increased cell firing in the hippocampus, which caused increases in burst firing and an increase in the number of cells firing in the ventral tegmental area, which then led to increased activity in the nucleus accumbens (the brain’s reward center). However, after the stressor was over, there was an opponent process that resulted in a much more prolonged period of inhibition in the nucleus accumbens, with associated decreases in psychomotor activity and reward seeking. The rodents lost their preference for sucrose and engaged in less intracranial self-stimulation, pressing a bar to stimulate the brain pleasurably. These and other effects suggest an analogy to anhedonia (loss of pleasure in activities that were previously enjoyed), which is a key component of human depression.
In related studies, after experiencing periods of inescapable shocks, rodents developed learned helplessness, failing to avoid the area where shocks were delivered even when an exit was readily available. Rodents who had learned helplessness showed inhibited firing of cells in the ventral tegmental area, less activity in the nucleus accumbens, and apparent anhedonia. This inhibition was mediated via messages from the infralimbic prefrontal cortex (the equivalent to the subgenual cingulate cortex in humans, important for motivation) to the amygdala and then the GABAergic ventral pallidum, which decreased the number of dopaminergic cells firing in the ventral tegmental area. Blocking the amygdala input to this inhibitory pathway reversed the low dopamine firing and the anhedonia-like behaviors.
The anesthetic ketamine (which has rapid-acting antidepressant effects in humans) produces an immediate reversal of the learned helpless behavior in the rodents and increases the number of dopamine cells firing in the ventral tegmental area. Ketamine administered directly into the nucleus accumbens induces long-term potentiation (enhanced synaptic responsivity) and reverses helpless behavior and the long-term depression of neural firing that is associated with it.
Thus, when an acute stressor is over and the opponent process emerges, or following long-term chronic stressors such as learned helplessness, the excitatory path to the ventral tegmental area is absent, while the inhibitory path to the ventral tegmental area (via the infralimbic prefrontal cortex, amygdala, and ventral pallidum) predominates. Ketamine is able to re-activate the activating pathway and increase activity in the ventral tegmental area and the nucleus accumbens, changes that are associated with the reversal of learned helplessness and anhedonia.
Editor’s Note: In the previous BNN, we reported researcher Scott Russo’s findings that input from the intralaminar nucleus of the thalamus was critical to the depression-like behaviors seen in a different animal model of depression, social defeat stress, where repeated exposure to defeat by a larger, more aggressive animal produces behaviors that resemble human depression. Here in Pitts’ research, learned helplessness is induced by inescapable shocks. Both models share the finding that firing decreases in the reward area of the brain (the nucleus accumbens). However, the key part of the brain driving the low levels of activity in the nucleus accumbens and the associated depression-like behavior appear to be different in these two different models. The intralaminar nucleus of the thalamus plays a key role in the social defeat stress model, while the infralimbic cortex and the amygdala play key roles in the learned helplessness model. These data together suggest that part of the reason depression differs from person to person may be because the illness can be driven by different brain areas as a result of different kinds of stressors.
Antidepressants and Ketamine Induce Resilience in Animals Susceptible to Depression-Like Behavior
 To study depression in humans, researchers look to rodents to learn more about behavior. Rodents who are repeatedly defeated by more aggressive animals often begin to exhibit behavior that resembles depression. At the 2014 meeting of the International College of Neuropsychopharmacology (CINP), researcher Andre Der-Avakian reported that in a recent study, repeated experiences of social defeat led to depressive behavior in a subgroup of animals (which he calls susceptible), but not in others (which he calls resilient). Among many biological differences, the resilient animals showed increases in neurogenesis in the dentate gyrus of the hippocampus.
To study depression in humans, researchers look to rodents to learn more about behavior. Rodents who are repeatedly defeated by more aggressive animals often begin to exhibit behavior that resembles depression. At the 2014 meeting of the International College of Neuropsychopharmacology (CINP), researcher Andre Der-Avakian reported that in a recent study, repeated experiences of social defeat led to depressive behavior in a subgroup of animals (which he calls susceptible), but not in others (which he calls resilient). Among many biological differences, the resilient animals showed increases in neurogenesis in the dentate gyrus of the hippocampus.
Chronic treatment of the susceptible animals with the selective serotonin reuptake inhibitor (SSRI) antidepressant fluoxetine or the tricyclic antidepressant desipramine, which both increase neurogenesis, also reversed the depressive behavior in about half of the animals. A single injection of the anesthetic ketamine (which has rapid-acting antidepressant effects in humans) reversed social avoidance behavior in about 25% of the animals. One depression-like symptom was anhedonia (loss of pleasure from previously enjoyed activities), which researchers measured by observing to what extent the animals engaged in intracranial self-stimulation, pressing a bar to stimulate the brain pleasurably. The effectiveness of the drugs in inducing resilient behavior was related to the degree of anhedonia seen in the animals. The drugs worked less well in the more anhedonic animals (those who gave up the intracranial stimulation more easily, indicating that they experienced less reward from it.)
Atypical Antipsychotics in Bipolar Depression
A limited number of atypical antipsychotics are approved by the Federal Drug Administration for the treatment of depression in patients with bipolar disorder. This is important to note, because the widely used traditional antidepressants that are highly effective in unipolar depression are not effective in bipolar depression. Here we review the status of the only three approved drug treatments for bipolar depression (olanzapine, quetiapine, and lurasidone) and highlight data on a promising new atypical antipsychotic, cariprazine.
Lurasidone (Latuda)
At the 2014 meeting of the International College of Psychopharmacology, researcher Joseph Calabrese reviewed the efficacy of the latest atypical antipsychotic to receive FDA approval for bipolar depression, lurasidone. In monotherapy, both low (20–60mg/day) and high doses (80–120mg/day) showed higher response rates (53% and 51%, respectively) than placebo (30%). When added to either lithium or valproate, lurasidone response (57%) again exceeded that of placebo (42%). Calabrese also indicated that all of the other secondary outcome measures were also statistically significant, including score on the Clinical Global Impressions scale for bipolar disorder, time to response, percentage of remitters, time to remit, score on the Hamilton Anxiety scale, and a patient rated depression scale (QIDS).
Lurasidone is also approved for schizophrenia at higher doses (up to 160mg/day). At least twice as much of the drug is absorbed when food is in the stomach, so it is recommended that patients take it one to two hours after dinner or after a snack of 350 calories or more. The drug has an excellent side effects profile, as it is weight- and metabolically- neutral (i.e. it does not increase blood glucose, cholesterol, or triglycerides).
Quetiapine (Seroquel)
The atypical antipsychotic quetiapine has been FDA-approved for bipolar depression for a number of years. It consistently performs better than placebo in bipolar depression, and unlike lurasidone, quetiapine is also FDA-approved for mania, as well as for long-term prevention of both manic and depressive episodes as an adjunct to either lithium or valproate. Quetiapine is also superior to placebo for prevention of both manic and depressive episodes as a monotherapy, but is not FDA-approved for this indication. A good target dose for bipolar depression is 300mg/day of the extended release preparation taken several hours prior to bed time. Higher doses of 400 to 800mg/night are used for mania and schizophrenia. Quetiapine is also FDA-approved as an adjunct to antidepressants in unipolar depression. The drug has sedative side effects, perhaps because of its potent antihistamine effects. It can also increase weight, glucose, and cholesterol slightly more than placebo.
Olanzapine and Fluoxetine
Olanzapine (Zyprexa) and a combined preparation of olanzapine and fluoxetine (Symbyax) are also approved for bipolar depression, but many guidelines suggest that these be considered secondary treatments because they are associated with weight gain and adverse metabolic effects.
Cariprazine Effective in Bipolar Depression and Mania
At the 2014 meeting of the International College of Neuropsychopharmacology, researcher Suresh Durgam presented a poster on the first study of the atypical antipsychotic cariprazine in bipolar depression. There have also been three positive placebo-controlled studies of the drug in mania. It is a dopamine D2 and D3 partial agonist, with greater potency at the D3 receptor than the atypical antipsychotic aripiprazole (Abilify). In the large placebo-controlled eight-week study, doses of 1.5mg/day were superior to placebo, but higher (3mg) and lower doses (0.75mg) were not.
Another poster presented by the same research group also reported that augmentation of antidepressants with cariprazine in unipolar depression had results that were significantly better than placebo.
Editor’s Note: While all atypical antipsychotics that have been tested for mania have antimanic efficacy (lurasidone has not been studied in mania), their antidepressant profiles differ considerably. Only the three atypical antipsychotics noted above (olanzapine/fluoxetine, quetiapine, and lurasidone) are FDA-approved for bipolar depression, and in light of recent findings, cariprazine is likely to follow soon.
The atypical antipsychotics NOT approved for bipolar depression include: aripiprazole (Abilify), risperidone (Risperidol), and ziprasidone (Geodon), with the first atypical antipsychotic clozapine and the most recent ones not yet formally tested as far as this editor is aware, including asenapine (Saphris), iloperidone (Fanapt), and paliperidone (Invega).
Only the atypical antipsychotics aripiprazole and quetiapine are FDA-approved as adjunctive treatments to antidepressants in unipolar depression, and cariprazine may soon be added to this list.
How the Chemicals in Marijuana Work in the Brain
Raphael Mechoulam, who first synthesized THC, the main ingredient in marijuana, gave the history of marijuana and its receptors in the central nervous system in a plenary talk at the 2014 meeting of the International College of Neuropsychopharmacology. In Syria hundreds of years ago the drug was named ganzigunnu, meaning “the drug that takes away the mind.” It has also been called azalla, meaning “hand of the ghost.” Among the 100 compounds in marijuana, the best-known ingredient is delta-9-tetrahydrocannabinol (delta-9 THC), which produces most of the actions of the drug. There is another active ingredient, cannabidiol (CBD), which has calming and anti-anxiety effects, but is present in very low levels.
The brain has cannabinoid receptors that respond to ingredients in marijuana in addition to other chemicals produced in the brain. They modulate calcium ions and decrease the release of many neurotransmitters.
THC acts at CB-1 receptors, producing the high. The CB-1 receptor is synthesized on demand, post-synaptically, and is transferred to the pre-synaptic terminal where it decreases calcium and transmitter release. Consistent with marijuana’s appetite-stimulating properties (“the munchies”), if the CB-1 receptor is blocked in animals, they lose their appetite and die of hunger.
There are also low levels of CB-2 receptors in the brain, whose activation does not cause a high, and whose levels may increase dramatically in pathological situations. Activation of the CB-2 receptor is anti-inflammatory and, in the same way that the immune system acts against foreign proteins, CB-2 acts as a protector against non-proteins.
CBD does not bind to any cannabinoid receptors, but its actions are blocked by cannabinoid antagonists.
There are two chemicals in the brain (endogenous ligands) that act at cannabinoid receptors—anandamide and 2-arachidonoylglycerol (2-AG). They are soluble only in lipids (not in water), and have never been given to people. In animals, 2-AG has neuroprotective effects, decreases the size of a stroke by 60%, and increases recovery from stroke.
Marijuana and CBD in particular have also had beneficial effects in people. Marijuana decreases the nausea and vomiting associated with chemotherapy in children, has anti-inflammatory effects in rheumatoid arthritis (decreasing inflammatory marker TNF alpha), and has anti-diabetes and anti-convulsant effects.
In 2012, researcher F. Markus Leweke and colleagues showed that CBD was about as effective as the atypical antipsychotic amisulpiride in alleviating the psychotic symptoms of schizophrenia. CBD’s other effects include reducing anxiety and improving psoriasis by increasing DNA methylation (Pucci et al. 2013).
It seems possible that some of these myriad effects of marijuana and endogenous ligands at CB receptors could be exploited for clinical therapeutics, as Mechoulam endorses, but when and how that will take place remains an unanswered question.
Editor’s Note: Despite all these potential positives of CBD, it should be noted that its levels are very low in marijuana, and that heavy smoking of marijuana has substantial adverse effects. These include low motivation, a doubling of the risk of psychosis, a hastening of the onset of bipolar disorder and schizophrenia, and cognitive impairment, as well as some changes in brain structure seen via magnetic resonance imaging (MRI). It may be becoming legal in many states, but is a bad idea for those at high risk for mood, anxiety, or bipolar disorders or for schizophrenia.
Antidepressant Use Dropping in Bipolar Disorder in Spain
In the clinic of researcher Eduard Vieta in Barcelona, a study was recently completed showing that antidepressant use in patients with bipolar disorder (where antidepressants are not effective) had dropped from around 50-60% in 2007 (in Baldessarini’s study) to about 30% in 2013 and 2014, and conversely lithium, anticonvulsants, and atypical antipsychotics, which have much more evidence of efficacy, were all used much more often, or about 60% of the time.
Editor’s Note: Hopefully these data from Spain will soon be matched by similar data in the US showing that evidenced-based treatments for bipolar depression are in fact being used instead of antidepressants, which can have adverse effects, such as switching into mania or cycle acceleration.
High Risk of Suicide Attempts in Bipolar Disorder with Substance Abuse
At the 2014 meeting of the International College of Neuropsychopharmacology, researcher Rieva et al. reported that 60% of bipolar patients with comorbid alcohol abuse have attempted suicide, and 48% of bipolar patients with cocaine abuse have attempted suicide. Thus, both of these comorbidities deserve specific attention and treatment. Unfortunately there are currently no Federal Drug Administration–approved drugs for bipolar patients with these comorbidities. The most promising treatments, based on data in patients with primary addictions, are the nutritional supplement N-acetylcysteine and topiramate, which have both performed better than placebo in studies of alcohol and cocaine abuse disorders.
A Symposium on High Risk Studies: Offspring of Parents with Bipolar Disorder
In a symposium at the 2014 meeting of the International College of Neuropsychopharmacology, four researchers shared insights on children who are at higher risk for bipolar disorder because they have a parent with the disorder.
Researcher John Nurnberger has been studying 350 children of parents with bipolar disorder in the US and 141 control children of parents with no major psychiatric disorder, following the participants into adolescence. He found a major affective disorder in 23.4% of the children with parents who have bipolar disorder and 4.4% of the controls. Of the at-risk children, 8.5% had a bipolar diagnosis versus 0% of the controls.
Nurnberger found that disruptive behavior disorders preceded the onset of mood disorders, as did anxiety disorders. These diagnoses predicted the later onset of bipolar disorder in the at-risk children, but not in the controls. A mood disorder in early adolescence predicted a substance abuse disorder later in adolescence among those at risk.
In genome-wide association studies, the genes CACNA1C and ODZ4 are consistently associated with risk of bipolar disorder, but with a very small effect size. Therefore, Nurnberger used 33 different gene variants to generate a total risk score and found that this measure was modestly effective in identifying relative risk of developing bipolar disorder. He hopes that using this improved risk calculation along with family history and clinical variables will allow better prediction of the risk of bipolar onset in the near future.
Researcher Ann Duffy reported on her Canadian studies of children who have a parent with bipolar disorder and thus are at high risk for developing the disorder. In contrast to the studies of Nurnberger et al. and many others in American patients, she found almost no childhood onset of bipolar disorder before late adolescence or early adulthood. She found that anxiety disorders emerge first, followed by depression, and then only much later bipolar disorder. Bipolar disorder occurred with comorbid substance abuse disorders in only about 10-20% of cases in 1975, but substance abuse increased to 50% of bipolar cases in 2005. The incidence of comorbid substance disorder and the year at observation correlated strongly, indicating a trend toward increased substance abuse over the 30-year period.
Duffy found that having parents who were ill as opposed to recovered was associated with a more rapid onset of mood disorder in the offspring, usually in early adulthood. Duffy emphasized the need to intervene earlier in children of parents with bipolar disorder, but this is rarely done in clinical practice. Read more









