GLP-1s Might Decrease the Incidence of Depression and Anxiety
Lisa O’Mary of WebMD wrote: “People taking a popular type of drug for weight loss or to manage diabetes have a lower likelihood of being newly diagnosed with depression or anxiety, according to an analysis of millions of people’s health records.
The findings were published this week by researchers from the electronic health record company Epic. Researchers looked for new diagnoses of depression or anxiety among people who started taking drugs from a class called GLP-1 agonists that can help manage blood sugar or treat obesity by mimicking hormone levels in the body that can affect appetite and blood sugar. Many people who take the drugs experience significant weight loss.
The researchers found that people with diabetes who started taking most versions of GLP-1 agonists were between 11% and 65% less likely to be newly diagnosed with depression than people with diabetes who didn’t take one of the drugs. The greatest reduction in likelihood of a new depression diagnosis was observed among people taking tirzepatide, which is sold under the brand names Mounjaro and Zepbound.”
Cannabidiol Reduces the Anxiety Induced by Simulated Public Speaking in Treatment-Naïve Social Phobia Patients
M M Bergamaschi, et al reported in Neuropsychopharmacology volume 36, pages 1219–1226 (2011) that the one of the ingredient in cannabis, the diol or cannabidiol (CBD), containing none of the usual THC which make up the vast majority of plant-based marijuana, reduces the anxiety Induced by simulated public speaking in treatment-naïve social phobia patients. They used “CBD (600?mg) in powder, ?99.9% pure (kindly supplied by STI-Pharm, Brentwood, UK and THC-Pharm, Frankfurt, Germany),… dissolved in corn oil.” This CBD has shown efficacy in other anxiety disorders and is FDA approved for one form of seizure disorder. This pure form of CBD is very expensive and usual preparations of available cannabis contain mostly THC and only minute amounts of CBD. Thus, the generalizability of these results to people using the widely available preparations of cannabis is extremely unlikely.
Mindfulness-Based Stress Reduction Equivalent to Escitalopram for Treatment of Anxiety Disorders
Hoge et al reported in JAMA Psychiatry (2022) that in a randomized clinical trial of 276 adults with anxiety disorders, 8-week treatment with mindfulness-based stress reduction (MBSR) was noninferior to escitalopram. The “MBSR is a manualized 8-week protocol with weekly 2.5-hour long classes, a day-long retreat weekend class during the fifth or sixth week, and 45-minute daily home practice exercises…. Qualified instructors taught the theory and practice of several forms of mindfulness meditation, such as breath awareness (focusing attention on the breath and other physical sensations), a body scan (directing attention to one body part at a time and observing how that body part feels), and mindful movement (stretching and movements designed to bring awareness to the body and increase interoceptive awareness)….Escitalopram was initiated at 10 mg daily orally and increased to 20 mg daily at week 2 if well tolerated or delayed if not.” The investigators concluded that “mindfulness-based stress reduction was a well-tolerated treatment option with comparable effectiveness to a first-line medication for patients with anxiety disorders.”
Study in Mice Suggests that Compound in Turmeric May Reduce Anxiety and Promote Resilience to Stress
 Chronic stress is a risk factor for the development of mood and anxiety disorders. Researchers have begun to focus on how to promote resilience to stress. Curcumin is a micronutrient found in turmeric that has anti-inflammatory and antidepressant effects and may promote such resilience.
Chronic stress is a risk factor for the development of mood and anxiety disorders. Researchers have begun to focus on how to promote resilience to stress. Curcumin is a micronutrient found in turmeric that has anti-inflammatory and antidepressant effects and may promote such resilience.
Researchers studying human depression often design studies to see how mice with chronic social defeat stress respond to various interventions. Mice who are repeatedly menaced by a larger mouse begin to show symptoms that resemble human depression, such as social avoidance, lack of interest in saccharin compared to plain water (a stand-in for loss of enjoyment or anhedonia in humans), and anxiety.
In a 2018 article in the journal Neuropsychopharmacology, researcher Antonio V. Aubry and colleagues described the effects of curcumin on mice undergoing chronic social defeat stress. Mice who were given a diet that consisted of 1.5% curcumin showed a 4.5-fold increase in resilience to social defeat stress, measured by their performance during a test of social interaction. Among the 129 mice in the study, 64% showed the increase in resilience, the remaining 36% did not respond to the curcumin diet and had the normal ‘depressed’ response. The mice who responded well to curcumin released less of the stress hormone corticosterone, and they also had lower levels of the inflammatory marker IL-6.
All of the mice on the curcumin diet showed reduced anxiety during tests that forced them to travel through open spaces (when they prefer to stay in more enclosed spaces or move along the edges of an enclosure).
Recent Cannabis Use Linked to Greater Symptoms of Anxiety and Mood Disorders and Less Response to Treatment
 In a 2018 systematic literature review published in the Journal of Clinical Psychiatry, researcher George Mamman and colleagues reported that across 12 studies of people with anxiety and mood disorders, participants who had used cannabis in the previous six months had more symptoms than those who had used less cannabis or no cannabis during that period.
In a 2018 systematic literature review published in the Journal of Clinical Psychiatry, researcher George Mamman and colleagues reported that across 12 studies of people with anxiety and mood disorders, participants who had used cannabis in the previous six months had more symptoms than those who had used less cannabis or no cannabis during that period.
The 12 studies reviewed included a total of 11,959 participants. Four studies looked at post-traumatic stress disorder (PTSD), one at panic disorder, five at bipolar disorder, and 2 at depressive disorder. In addition to finding that recent cannabis use was associated with greater symptoms, the authors of the review also found that in 10 of the 12 studies, recent cannabis use was associated with less symptom improvement in response to treatment for bipolar disorder, depression, and PTSD; including both medication and psychotherapy.
In bipolar disorder, cannabis use was associated with greater symptom severity. Cannabis use for more than one year was linked to more recurrences of mania and shortened time to a recurrence. Compared to participants with no prior use of cannabis, those with a cannabis use disorder had more depressive symptoms, including sleep troubles and loss of interest in activities one had previously enjoyed.
In PTSD, any cannabis use at the beginning of the analysis period and sustained use of cannabis over time were both linked to greater symptom severity in the four months following the beginning of the analysis.
Mammen and colleagues cautioned that these results are limited based on the differences in measurements across the 12 studies, the inpatient populations under study, and the uncontrolled nature of the cannabis the participants accessed on their own time. However, the authors suggest that the findings may inform patients’ and doctors’ conversations about whether or not to use cannabis.
Treatment Approaches to Childhood-Onset Treatment-Resistant Bipolar Disorder
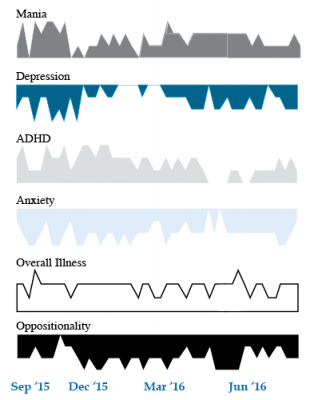
Dear readers interested in the treatment of young children with bipolar disorder and multiple other symptoms: In 2017, BNN Editor Robert M. Post and colleagues published an open access paper in the journal The Primary Care Companion for CNS Disorders titled “A Multi-Symptomatic Child: How to Track and Sequence Treatment.” The article describes a single case of childhood-onset bipolar disorder shared with us via our Child Network, a research program in which parents can create weekly ratings of their children’s mood and behavioral symptoms, and share the long-term results in graphic form with their children’s physicians.
Here we summarize potential treatment approaches for this child, which may be of use to other children with similar symptoms.
We present a 9-year-old girl whose symptoms of depression, anxiety, attention-deficit hyperactivity disorder (ADHD), oppositional behavior, and mania were rated on a weekly basis in the Child Network under a protocol approved by the Johns Hopkins School of Medicine Institutional Review Board. The girl, whose symptoms were rated consistently for almost one year, remained inadequately responsive to lithium, risperidone, and several other medications. We describe a range of other treatment options that could be introduced. The references for the suggestions are available in the full manuscript cited above, and many quotes from the original article are reprinted here directly.
As illustrated in the figure below, after many weeks of severe mania, depression, and ADHD, the child initially appeared to improve with the introduction of 4,800 micrograms per day of lithium orotate (a more potent alternative to lithium carbonate that is marketed as a dietary supplement), in combination with 1 mg per day of guanfacine, and 1 mg per day of melatonin.
Despite continued treatment with lithium orotate (up to 9,800 micrograms twice per day), the patient’s oppositional behavior worsened during the period from November 2015 to March 2016, and moderate depression re-emerged in April 2016. Anxiety was also generally less severe from December 2015 to July 2016, and weekly ratings of overall illness remained in the moderate severity range (not illustrated).
In June 2016, the patient began taking risperidone (maximum dose 1.7 mg/day) instead of lithium, and her mania improved from moderate to mild. There was little change in her moderate but fluctuating depression ratings, but her ADHD symptoms got worse.
The patient had been previously diagnosed with bipolar II disorder and anxiety disorders including school phobia, generalized anxiety disorder, and obsessive compulsive disorder.
Given the six weeks of moderate to severe mania that the patient experienced in October and November 2015, she would meet criteria for a diagnosis of bipolar I disorder.
Targeting Symptoms to Achieve Remission
General treatment goals would include: mood stabilization prior to use of ADHD medications, a drug regimen that maximizes tolerability and safety, targeting of residual symptoms with appropriate medications supplemented with nutraceuticals, recognition that complex combination treatment may be necessary, and combined use of medications, family education, and therapy.
Mood Stabilizers and Atypical Antipsychotics to Maximize Antimanic Effects
None of the treatment options in this section are approved by the US Food and Drug Administration for use in children under 10 years of age, so all of the suggestions are “off label.” Further, they may differ from what other investigators in this area of medicine would suggest, especially since evidence-based medicine’s traditional gold standard of randomized placebo-controlled clinical trials is impossible to apply here, given the lack of research in children with bipolar disorder.
As we share in the original article, reintroducing lithium alongside risperidone could be effective, as “combinations were more effective than monotherapy in a study [by] Geller et al. (2012), especially when they involved an atypical antipsychotic such as risperidone. This might include the switch from lithium orotate to lithium carbonate,” the typical treatment for bipolar disorder, on which more research has been done. “Combinations of lithium and valproate were also more effective than either [drug alone]…in the studies of Findling et al. (2006),” and many patients needed stimulants in addition.
“Most children also needed combinations of mood stabilizers (lithium, carbamazepine, valproate) in the study [by] Kowatch et al. (2000).” In a 2017 study by Berk et al. of patients hospitalized for a first mania, randomization to lithium for one year was more effective than quetiapine on almost all outcome measures.
Targeting ADHD
“[The increased] severity of [the child’s] ADHD despite improving mania speaks to the…utility of adding a stimulant to the regimen that already includes…guanfacine,” which is a common non-stimulant treatment for ADHD. “This would be supported by the data of Scheffer et al. (2005) that stimulant augmentation for residual ADHD symptoms does not [worsen] mania, and that the combination of a stimulant and guanfacine may have more favorable effects than stimulants alone.”
However, the consensus in the field is that mood stabilization should be achieved first, before low to moderate (but not high) doses of stimulants are added. “Thus, in the face of an inadequate response to the lithium-risperidone combination in this child, stimulants could be deferred until better mood stabilization was achieved.”
Other Approaches to Mood Stabilization and Anxiety Reduction
“The anticonvulsant mood stabilizers (carbamazepine, lamotrigine, and valproate) each have considerable mood stabilizing and anti-anxiety effects, at least in adults with bipolar disorder. With inadequate mood stabilization of this patient on lithium and risperidone, we would consider the further addition of lamotrigine.
Lamotrigine appears particularly effective in adults with bipolar disorder who have a personal history and a family history of anxiety (as opposed to mood disorders), and it has positive open data in adolescents with bipolar depression and in a controlled study of maintenance (in teenagers 13–17, but not in preteens 10–12) (Findling et al. 2015). With better mood stabilization, anxiety symptoms usually diminish…, and we would pursue these strategies [instead of using] antidepressants for depression and anxiety in young children with bipolar disorder.”
“Carbamazepine appears to be more effective in adults with bipolar who have [no] family history of mood disorders,” unlike lithium, which seems to work better in people who do have a family history of mood disorders.
“While the overall results of oxcarbazepine in childhood mania were negative, they did exceed placebo in the youngest patients (aged 7–12) as opposed to the older adolescents (13–18) (Wagner et al. 2006).
“There are long-acting preparations of both carbamazepine (Equetro) and oxcarbazepine (Oxtellar) that would allow for all nighttime dosing to help with sleep and reduce daytime side effects and sedation. Although data [on] anti-manic and antidepressant effects in adults are stronger for carbamazepine than oxcarbazepine,” there are good reasons to consider oxcarbazepine. First, there is the finding mentioned above that oxcarbazepine worked best in the youngest children. Second, there is a lower incidence of severe white count suppression on oxcarbazepine. Third, it has less of an effect on liver enzymes than carbamazepine. However, low blood sodium levels are more frequent on oxcarbazepine than carbamazepine.
Other Atypical Antipsychotics That May Improve Depression
Children Who Are Bullied Have Poorer Mental Health
A 2017 study of twins between the ages of 11 and 16 found that being bullied around age 11 caused anxiety, depression, hyperactivity and impulsivity, inattention, and conduct problems, some of which lasted for years. Participants recorded their experiences with physical or verbal bullying, social manipulation, and property attacks (trying to break one’s belongings, for example).
The effects of bullying decreased over time. The bullied children were still significantly more anxious than their non-bullied twins two years later, but this difference faded by the five-year mark. However, paranoid thoughts and cognitive disorganization did persist for 5 years.
The twin study design helped researchers zone in on the causal effect bullying might have on the children’s mental health, rather than other factors the twins shared, such as genetics or family environment. The study included 11,108 twins born in England and Wales.
The research by Timothy Singham and colleagues was published in the journal JAMA Psychiatry. Interestingly, the researchers found that prior mental health difficulties increased children’s likelihood of being bullied, such that being bullied could be considered a symptom of preexisting vulnerabilities. Singham and colleagues suggest that in addition to interventions to reduce bullying and address familial factors that might make children susceptible to bullying, children should also be taught resilience skills.
Clinical Vignettes from Dr. Elizabeth Stuller
 Dr. Elizabeth Stuller, a staff psychiatrist at the Amen clinics in Washington, DC and CEO of private practice Stuller Resettings in Baltimore, MD, provided this editor (Robert M. Post) with several interesting anecdotal observations based on her wide clinical experience with difficult-to-treat mood disordered patients.
Dr. Elizabeth Stuller, a staff psychiatrist at the Amen clinics in Washington, DC and CEO of private practice Stuller Resettings in Baltimore, MD, provided this editor (Robert M. Post) with several interesting anecdotal observations based on her wide clinical experience with difficult-to-treat mood disordered patients.
- Stuller has used low-dose asenapine (Saphris), e.g. half a pill placed under the tongue, for depressed patients with alcohol use problems who have trouble getting to sleep. She has also used asenapine for rapid calming of agitated patients in her office.
- Stuller has also had success with the use of the atypical antipsychotic drug brexpiprazole (Rexulti) for patients with bipolar depression and low energy. She typically uses 0.5 mg/day for women and 1 mg/day for men. Stuller finds that there is little weight gain or akathisia with brexpiprazole.
- She has had success with the drug Nuedexta, which is a combination of dextromethorphan and quinidine and is approved for the treatment of sudden uncontrollable bouts of laughing or crying, known as pseudobulbar affect, which can occur as a result of neurological conditions or brain injuries. It is a combination of an NMDA antagonist and a sigma receptor agonist. Stuller starts with the 20mg dextromethorphan/10 mg quinidine dose once a day and increases to twice a day in week two. She finds it useful for behavioral effects of traumatic brain injury (TBI), anxiety resulting from the use of synthetic marijuana (sometimes called spice), and psychosis not otherwise specified. Stuller also finds that some patients appear to respond well to Nuedextra but not minocycline, or vice versa.
Editor’s Note: Note that these are preliminary clinical anecdotes conveyed in a personal communication, and have not been studied in clinical trials, thus should not be relied upon in the making of medical decisions. All decisions about treatment are the responsibility of a treating physician.
In Animals, Exposure to High Fat Diet During Pregnancy Can Affect Offspring’s Neurological Development
New research in non-human primates suggests that exposure to a high fat diet during pregnancy and in early development prior to weaning can increase the offspring’s propensity for anxiety later in life.
The new research echoes 2010 findings about rats. Researcher Staci D. Bilbo and colleagues reported in the journal of the Federation of American Societies for Experimental Biology that in rats, a high fat diet during pregnancy and lactation led to offspring with greater body weight, increased inflammation, and problems with anxiety and spatial learning. Switching to a standard diet after weaning did not eliminate these outcomes.
The recent research by Jacqueline R. Thompson and colleagues, published in the journal Frontiers in Endocrinology in July 2017, suggests that maternal nutrition in the primate during pregnancy and lactation can have long-lasting effects on offspring’s neurological development, altering the brain and endocrine system. These changes occurred even if the offspring began a normal diet after weaning.
65 female Japanese macaques were divided into two groups, one that received a high-fat diet and one that received a normal diet. In the offspring of mothers who ate a high-fat diet, the researchers found impaired development of neurons containing serotonin. The offspring of the high-fat diet group also showed behavioral alterations such as increased anxiety.
The high rates of obesity in the US and other developed nations make these findings particularly important. The researchers suggest that 64% of women in the US who are of reproductive age are overweight, and 35% are obese. Co-author Elinor Sullivan suggested that the findings from the study could motivate mothers to make healthy nutritional decisions, not only for themselves but for their children as well.
Link Clarified Between Gut Microbes and Emotions
A 2017 article in the journal Microbiome suggests that gene-regulating molecules called microRNAs in the brain may be the link between microbes in the gut and emotions.
The research by Alan E. Hoban and colleagues looked at mice raised in a sterile, microbe-free environment. These mice had fewer anxiety-like behaviors than mice raised among the usual bacteria, viruses, and fungi. This finding implies that the microbiome—the trillions of microbes that live in and around our bodies—affects brain functions. In this case, the affected regions were the prefrontal cortex and the amygdala, which both play a role in the detection and response to fearful stimuli. These regions showed alterations in the level of microRNAs present.
When Hoban and colleagues introduced microbes into the animal’s systems, some microRNAs did not bounce back, suggesting that there may be a crucial window early in life when the presence of microbes is needed for the brain to develop normally.
In general, this research shows that microRNAs are key to understanding the link between the microbiome and the brain.