Potential of Environmental Enrichment to Prevent Transgenerational Effects of Paternal Trauma
Gapp, K. et al. wrote about the “Potential of Environmental Enrichment to Prevent Transgenerational Effects of Paternal Trauma” in Neuropsychopharmacol 41, 2749–2758 (2016).
They “used a mouse model of unpredictable maternal separation combined with unpredictable maternal stress (MSUS) to examine the consequences of traumatic stress on coping behaviors in adulthood and across generations, and the potential contribution of (glucocorticoid receptors) GR. We show that MSUS affects avoidance behaviors and learning in aversive environments in exposed fathers and their male offspring. This is associated with an increase in GR expression in the hippocampus, and with decreased DNA methylation of GR promoter in the hippocampus and in germ cells. We show that transmission of the effects of paternal trauma can be prevented by paternal (environmental enrichment) EE, suggesting a reversibility of these effects.”
Editors Note: Dad’s early environmental adversity alter his response to traumatic stress as an adult, and this can be passed to the next generation via epigenetic changes in DNA methylation, histone and microRNA chemical changes persisting in sperm. If the dad with early life adversity is housed in an enriched environment, he does not have the altered response to stress or the changes in GR, and his offspring do not have the transgenerational alterations in stress responsively. This could probably happen in people if we could only figure out to super good environmental enrichment in those having early life adversity. Having lots of stress as a neonate and then being adopted out to wonderful foster family could be the basis for a naturalistic study of this sort of result.
Neurotransmitters Can Also Function As Epigenetic Marks
The most common epigenetic marks involve methylation of DNA (which usually inhibits gene transcription) and the acetylation and methylation of histones. Acetylation opens or loosens the winding of DNA around the histones and facilitates transcription, while methylation of histones leaves the DNA tightly wound and inhibits transcriptional activation.
Researcher Ashley E. Lepack and colleagues have identified a surprising type of epigenetic mechanism involving neurotransmitters. They report in a 2020 article in the journal Science that neurotransmitters such as serotonin and dopamine can act as epigenetic marks. Dopamine can bind to histone H3, a process called called dopaminylation (H3Q5dop). In rats undergoing withdrawal from cocaine, Lepack and colleagues found increased levels of H3Q5dop in dopamine neurons in a part of the midbrain called the ventral tegmental area (VTA), a part of the brain’s reward system. When the investigators reduced H3Q5dop, this decreased dopamine release in the reward area of the brain (the nucleus accumbens) and reduced cocaine seeking. Thus, dopamine can be both an important transmitter conveying messages between neurons and a chemical mark on histones that alters DNA binding and transcriptional regulation.
Researcher Jean-Antoine Girault provided commentary on the article by Lepack and colleagues, writing that “[t]he use of the same monoamine molecule as a neurotransmitter and a histone modification in the same cells illustrates that evolution proceeds by molecular tinkering, using available odds and ends to make innovations.”
Editor’s Note: Epigenetic marks may remain stable and influence behavior over long periods of time. They are involved in the increased reactivity or sensitization to repeated doses of cocaine through DNA methylation. Such sensitization can last over a period of months or longer. If the methylation inhibitor zebularine is given, animals fail to show sensitization. Now a newly identified epigenetic process, dopaminylation, is found to alter histones and is associated with long-term changes in cocaine-seeking.
The clinical message for a potential cocaine user is ominous. Cocaine not only creates a short-term “high,” but its repeated use rewires the brain not only at the level of changes in neurotransmitter release and receptor sensitivity, but also at the genetic and epigenetic level, changes that could persist indefinitely.
The sensitization to motor hyperactivity and euphoria that occur with cocaine use can progress to paranoia and panic attacks and eventually even seizures (through a process known as kindling).
The dopaminylation of histones in the VTA could lead to persistent increases in drug craving and addiction that may not be easily overcome. Thus, the appealing short-term effects of cocaine can spiral into increasingly adverse behaviors and drug-seeking can become all consuming. While these adversities do not emerge for everyone, the best way to ensure that they do not is to avoid cocaine from the start.
Manic episodes that include a feeling of invincibility, increased social contacts, and what the DSM-5 describes as “excessive involvement in pleasurable activities that have a high potential for painful consequences” are a time that many are at risk for acquiring a substance problem. For the adolescent who has had a manic episode, ongoing counseling about avoiding developing this type of additional long-term, difficult-to-treatment psychiatric illness could be lifesaving. Describing the epigenetic consequences of substance use may or may not be helpful, but may be worth a try.
Environment Can Leave “Molecular Scars” Via Epigenetic Processes

A 2020 review article by researchers Julia Richetto and Urs Meyer in the journal Biological Psychiatry provides a good overview of the role epigenetic modifications play in schizophrenia and related disorders.
The article provides a powerful understanding of how the environment can induce long-lasting changes in the structure of DNA (not only in schizophrenia, but also in bipolar disorder). This process, known as epigenetics, can have life-long influences on brain chemistry and behavior, and remarkably, some of these epigenetic changes can even be transmitted to the next generation.
While the sequence of DNA that one inherits from one’s parents does not change over the course of one’s life, what can change is how loosely or tightly the DNA is wound around proteins called histones, making it easier or harder to transcribe the genetic material held there. The addition of a methyl group to DNA usually inhibits transcription, while the addition of an acetyl group to histones usually facilitates transcription. These alterations in the shape of the DNA that result from environmental exposures or behavior can be passed on through generations.
Richetto and Meyer describe these chemical changes to DNA as “molecular scars,” which are left when environmental stress occurs during sensitive developmental periods. For example, patients with schizophrenia who experienced stressors in early life have higher levels of the enzyme histone deacetylase than patients who had stress or trauma later in life. Histone deacetylase would remove the acetyl groups on histones, which would inhibit gene transcription.
Other factors that have been implicated in epigenetic modifications in schizophrenia, such as DNA methylation of key developmental pathways, include pre- or post-natal stress, a challenge to a mother’s immune system during pregnancy, pre- and post-natal nutrition, exposure to drugs or toxic substances, and cannabis use in adolescence.
Richetto and Meyer suggest that epigenetics may explain why schizophrenia (and we would add bipolar disorder) can differ so much across individuals, and may help researchers and clinicians determine how best to treat different individuals.
Editor’s Note: This editor has written about how epigenetic changes can mediate sensitization to the recurrence of life stressors, episodes of mood disorder, and bouts of substance abuse, each of which can drive illness exacerbation and progression in bipolar disorder (see the 2016 article by Robert M. Post in the journal Bipolar Disorders, “Epigenetic basis of sensitization to stress, affective episodes, and stimulants: implications for illness progression and prevention”).
The chemical changes to our DNA, histones, and microRNA emphasize how important it is to begin long-term preventative treatment starting after a first episode of mania. This not only helps limit episode recurrence and the accumulation of stressors and bouts of substance use that can cause illness deterioration, but also limit the placement of these “molecular scars” on our DNA. The key to treating bipolar disorder is: prevent episodes, protect the person and the brain.
Risk Gene for Bipolar Disorder Implicated in Depressed Behaviors and Abnormal Firing of GABA Neurons
At a 2018 scientific meeting and in a 2017 article in the journal PNAS, researcher Shanshan Zhu and colleagues reported that mice genetically engineered to lack the protein Ankyrin-G in certain neurons showed increases in depression- and mania-like behavior after being exposed to defeat stress (by repeatedly being placed in physical proximity to a larger, more aggressive mouse), which is often used to model human depression.
The researchers targeted the gene ANK3, which is responsible for the production of Ankyrin-G, and has been linked to bipolar disorder in genome-wide association studies. By manipulating the gene, they could eliminate Ankyrin-G in pyramidal neurons in the forebrain, a region relevant to many psychiatric disorders. Pyramidal neurons perform key brain functions, sending nerve pulses that lead to movement and cognition.
The missing Ankyrin-G affected sodium channels (which allow for the flow of sodium ions in and out of cells) and potassium channels. The neurochemical GABA (which typically inhibits nerve impulses) was also dysregulated, resulting in the kind of disinhibition seen in psychosis. Mice showed dramatic behavioral changes ranging from hyperactivity to depression-like behavior (e.g. giving up in a forced swimming test). The hyperactivity decreased when the mice were given treatments for human mania, lithium or valproic acid.
While mutations in the ANK3 gene may disturb sodium channels, another gene linked to depression and bipolar disorder, CACNA1C, affects calcium channels.
In a related study by researcher Rene Caballero-Florán and colleagues that was also presented at the meeting, mice were genetically engineered in such a way that interactions between Ankyrin-G and GABA Type A Receptor-Associated Protein (GABARAP) were disrupted, leading to deficits in inhibitory signaling. These deficits were partially corrected when the mice were treated with lithium.
The study by Caballero-Florán and colleagues used mice with a mutation known as W1989R in the ANK3 gene. Through a program that examines the genes of people with bipolar disorder, the researchers also identified a family with this genetic mutation, including a patient with type I bipolar disorder with recurrent mania and depression who has responded well to lithium treatment.
Nimodipine Decreases Frontal and Parietal Cortical Activity During Working Memory in Healthy Subjects
At a recent scientific meeting, researcher Kristin Bigos and colleagues described the effects of nimodipine, a treatment for brain hemorrhage, on the brain during working memory tasks. Nimodipine is a dihydropyridine L-type calcium channel blocker. Calcium channel blockers prevent calcium from entering cells in the heart and blood vessel walls, and they are often used to treat high blood pressure.
Nimodipine acts on the CACNA1C calcium influx gene. Certain genetic variations in this gene (particularly the rs1006737 A allele) have been linked to vulnerability to bipolar disorder, schizophrenia, depression, and autism. Carriers of the risk allele also have higher CACNA1C mRNA expression in the dorsolateral prefrontal cortex and exhibit more activity in the frontal and parietal regions of the brain during working memory tasks, suggesting inefficient brain processing in these regions. Bigos and colleagues found that 60mg/day of nimodipine decreased frontal and parietal cortical activity by 39.1% and 42.8%, respectively, during a working memory task, suggesting that nimodipine improved the efficiency of memory processing. Nimodipine’s positive effects were greater in those participants who had the CACNA1C risk allele.
Editor’s Note: Using a placebo-controlled off-on-off-on study design (meaning patients took placebo for a period, then nimodipine, then placebo again and nimodipine again), this editor (Robert M. Post), Peggy J. Pazzaglia and colleagues found that nimodipine had positive effects in both mania and depression in patients with bipolar disorder (described in the 2008 book Treatment of Bipolar Disorder: A Casebook for Clinicians and Patients by Robert M. Post and Gabriele S. Leverich). In a large randomized study of patients with bipolar disorder presented by Haroon R. Chaudhry at the 2010 meeting of the Society of Biological Psychiatry, lithium was associated with about a 50% response rate while the combination of lithium and nimodipine was associated with a 73% response rate.
It remains to be seen whether people with bipolar disorder who have the CACNA1C risk gene would respond better to nimodipine than those without the risk gene, and whether it would improve working memory more in the subgroup with the risk gene.
Phthalates in Plastics and Creams Cause Epigenetic Changes to Sperm
A recent study suggests that chemicals called phthalates that are used to make plastic flexible and to improve the texture of lotions, creams, and powders have effects on human sperm. Phthalates have become common in our environment since the invention of plastics, and most people have detectable levels of phthalate metabolites in their bodies.
The study, published by Haotian Wu and colleagues in the journal Human Reproduction in 2017, measured DNA methylation in a group of men’s sperm and compared this to levels of phthalate metabolites in the men’s urine.
DNA methylation changes the structure of a DNA strand. Extra methyl groups are attached to the strand, affecting the way it is transcribed, even though the inherited genetic sequence on the DNA strand remains the same. Changes like these to the structure of DNA and histones, which give DNA its helix shape, are known as epigenetic changes.
Wu and colleagues found 131 regions of DNA methylation in the men’s sperm that they could link to at least one of the phthalate metabolites found in the men’s urine.
Sperm takes 72 days to mature. Wu and colleagues suggest that exposure to phthalates in plastics or personal care products during this period may cause alterations to sperm, which could potentially affect the ease of conception or the development of potential offspring. The changes the researchers observed affected genes related to growth, development, and cellular function and maintenance.
In addition to chemical exposure, stressors and drug use can also bring about epigenetic changes to sperm. A father’s offspring may then have altered risk of drug use or other behaviors as a result of these epigenetic changes.
Phthalates, which can disrupt the endocrine system, have previously been found to alter men’s hormone levels and to hurt sperm quality. This is the first study to find that in people, phthalate concentrations measured before conception are associated with DNA methylation in sperm. This was a fairly small study in 48 men, and it remains to be studied whether the changes to sperm affect the offspring’s prenatal and early childhood development.
In addition to their presence in flexible plastics, phthalates may also be found in products such as shaving cream, shampoo, soaps, and detergents.
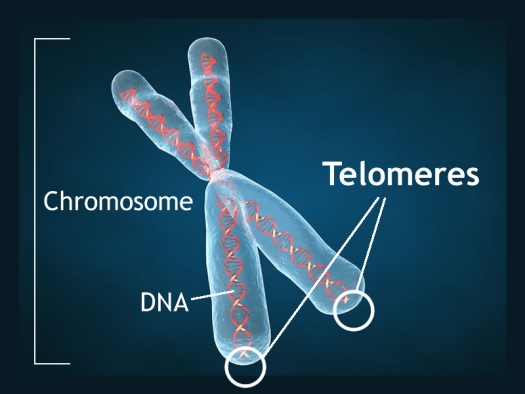
Short Telomeres Associated with Family Risk of Bipolar Disorder
Telomeres are bits of genetic material at the end of each strand of DNA that protect chromosomes as they replicate. Short telomeres have been linked to aging and a variety of medical and psychiatric diseases. Stress and depressive episodes can shorten telomeres, while treatment with lithium can lengthen them.
Telomere length is a heritable trait, and a 2017 study by researcher Timothy R. Powell and colleagues suggests that shorter telomeres are a familial risk factor for bipolar disorder.
The study, published in the journal Neuropsychopharmacology, compared the telomere lengths of 63 people with bipolar disorder, 74 of their immediate relatives (49 of whom had no lifetime psychiatric illness, while the other 25 had a different mood disorder), and 80 unrelated people with no psychiatric illness. The well relatives of the people with bipolar disorder had shorter telomeres than the unrelated healthy volunteers.
Relatives (both well and not) and people with bipolar disorder who were not being treated with lithium both had shorter telomeres than people with bipolar disorder who were being treated with lithium.
Another finding was that longer telomeres were linked to greater volume of the left and right hippocampus, and improved verbal memory on a test of delayed recall. This study provides more evidence that taking lithium increases the volume of the hippocampus and has neuroprotective benefits for people with bipolar disorder.
Levels of Amino Acid Proline Interact with COMT Genotype to Affect Negative Symptoms
 In a 2016 article, researcher Catherine L. Clelland and colleagues reported that a patient’s levels of the amino acid proline interact with their genetic profile to influence the seriousness of their negative symptoms. Negative symptoms of schizophrenia and bipolar disorder include flat affect and lack of volition and can be some of the hardest symptoms to treat.
In a 2016 article, researcher Catherine L. Clelland and colleagues reported that a patient’s levels of the amino acid proline interact with their genetic profile to influence the seriousness of their negative symptoms. Negative symptoms of schizophrenia and bipolar disorder include flat affect and lack of volition and can be some of the hardest symptoms to treat.
High levels of proline in the central nervous system have been linked to schizophrenia. Proline is a precursor to the neurotransmitter glutamate, and high proline levels have been found to alter glutamate and dopamine signaling in mice. This is one of the factors affecting negative symptoms.
The other factor affecting negative symptoms is the COMT gene. The enzyme catechol-o-methlyl transferase (COMT) metabolizes dopamine in the prefrontal cortex. There are several common versions of the gene for COMT. The most efficient is known as val-158-val, identifying that the gene has two valine amino acids at position 158. People with high proline levels and the val-158-val version of the COMT gene had fewer negative symptoms than people with high proline levels and another version of the gene, val-158-met (indicating one valine and one methionine amino acid at position 158).
Clelland and colleagues hypothesized that high proline levels may actually counteract the dopamine shortages common in the prefrontal cortex in people with the val-158-val genotype of COMT, which is more efficient at breaking down dopamine in this region.
The mood stabilizer valproate increases proline levels. In the study, which was published in Translational Psychiatry, people with schizophrenia and the val-val genotype had fewer negative symptoms when treated with valproate than those with the val-met genotype who received the same treatment.
Early Cannabis Use and BDNF Gene Variant Increase Psychosis Risk
 Normal variations in genes can affect risk of mental illness. One gene that has been implicated in psychosis risk is known as BDNF. It controls production of brain-derived neurotrophic factor, a protein that protects neurons and is important for learning and memory. Another important gene is COMT, which controls production of the enzyme catechol-O-methyltransferase, which breaks down neurotransmitters such as dopamine in the brain.
Normal variations in genes can affect risk of mental illness. One gene that has been implicated in psychosis risk is known as BDNF. It controls production of brain-derived neurotrophic factor, a protein that protects neurons and is important for learning and memory. Another important gene is COMT, which controls production of the enzyme catechol-O-methyltransferase, which breaks down neurotransmitters such as dopamine in the brain.
Several forms of these genes appear in the population. These normal variations in genes are known as polymorphisms. Certain polymorphisms have been linked to disease risk. A study by Anna Mané and colleagues published in the Journal of Psychiatric Research in 2017 explored links between COMT and BDNF polymorphisms, cannabis use, and age at first episode of psychosis.
Mané and colleagues found that among 260 Caucasians being treated for a first episode of psychosis, the presence of a BDNF polymorphism known as val-66-met and a history of early cannabis use were associated with younger age at psychosis onset.
The val-66-met version of BDNF occurs in 25-35% of the population. It functions less efficiently than a version called val-66-val.
The researchers also found that males were more likely to have used cannabis at a young age.
Editor’s Note: In the general population, marijuana use doubles the risk of developing psychosis. Previous data had indicated that the risk was higher for those with a COMT polymorphism known as val-158-val that leads to more efficient metabolism of dopamine in the prefrontal cortex. The resulting deficits in dopamine increase vulnerability to psychosis compared to people with the val-158-met version of the COMT gene.
The new study by Mané and colleagues suggests that a common form of BDNF may be associated with an earlier onset of psychosis. Bottom line: Pot is dangerous for young users and can induce psychosis, particularly in people at genetic risk. Pot may be legal in many places, but heavy use in young people remains risky for mental health and cognitive functioning.
The company Genomind offers genetic testing for BDNF and COMT variants as part of a routine panel.
In Rats, Mother’s Exercise Habits Affect Those of Offspring
 A recent study suggests that when a mother rat exercises during pregnancy, her offspring will exercise more too.
A recent study suggests that when a mother rat exercises during pregnancy, her offspring will exercise more too.
In the study, published by Jesse D. Eclarinel and colleagues in The FASEB Journal, pregnant mother rats were placed in cages that each contained an exercise wheel. One group had access to a working wheel on which they could run. The other group had the same wheel, but it was locked so that they couldn’t use it for running. Daughters of the rats who ran during pregnancy ran more in adulthood (both at 60 days and 300 days after birth) than daughters of the rats who couldn’t run during pregnancy.
While it is a mystery why this occurs, it is consistent with other data about the ways that a parent’s experiences can influence the next generation, even when the offspring don’t grow up with the parents.
For example, father rats conditioned to associate a specific smell with fear of an electric shock have offspring that also fear that smell (but not other smells).
Drug use is another example. Father rats given access to cocaine have offspring that are less interested in cocaine. Interestingly, father rats exposed to marijuana have offspring that are more interested in opiates.
Experiences with drugs or stress are thought to affect the next generation via ‘epigenetic’ marks on ova or sperm. These marks change the way DNA is packaged, with long-lasting effects on behavior and chemistry. Most marks from a mother’s or father’s experiences are erased at the time of conception, but some persist and affect the next generation.
The nature versus nurture debate is getting more and more complicated. Parents can influence offspring in a number of ways: 1) genetics; 2) epigenetics in the absence of contact between parent and offspring after birth; 3) epigenetic effects of behavioral contact—that is, parents’ caring and warmth versus abuse and neglect can affect offspring’s DNA expression too. All these are in addition to any purely behavioral influence a parent may have on their offspring via discipline, teaching, being a role model, etc.
Editor’s Note: The moral of the story is, choose your parents wisely, or behave wisely if you yourself become a parent.