Oxytocin Improves Facial Expressiveness in People with Autism
 In a new study by Keiho Owada and colleagues, 18 people with autism spectrum disorders had more neutral facial expressions and fewer surprised expressions than 17 typically developing people while interacting socially. Oxytocin, a hormone that promotes social bonding, was delivered to the autism group via a nasal spray for six weeks, and made the faces of the people with autism more expressive. Oxytocin also improved their reciprocity in social interactions and increased activity in the dorsomedial prefrontal cortex, as observed via functional magnetic resonance imaging (fMRI).
In a new study by Keiho Owada and colleagues, 18 people with autism spectrum disorders had more neutral facial expressions and fewer surprised expressions than 17 typically developing people while interacting socially. Oxytocin, a hormone that promotes social bonding, was delivered to the autism group via a nasal spray for six weeks, and made the faces of the people with autism more expressive. Oxytocin also improved their reciprocity in social interactions and increased activity in the dorsomedial prefrontal cortex, as observed via functional magnetic resonance imaging (fMRI).
The study suggests not only that oxytocin can normalize facial expressions, but also that the counting of facial expressions on videos of social interactions can be used as a measure of social symptoms of autism. The research was presented at the 2016 meeting of the Society of Biological Psychiatry.
Animal Studies Suggest That Oxytocin May Treat Addictions
Oxytocin, the hormone that promotes emotional bonding, also regulates a variety of behaviors. Two recent studies suggest that in rats, an injection of oxytocin can prevent drug-seeking behavior.
In the first study, researcher Gary Aston-Jones found that oxytocin reduced the rats’ interest in methamphetamine. The effect was strongest in the rats that started out with the strongest interest in the methamphetamine.
In the second study, researcher Luyi Zhou and colleagues determined that oxytocin also reduced cocaine-seeking behavior in rats. In addition, the oxytocin reversed changes in the brain’s glutamate signaling pathway that were caused by cocaine use.
Both studies, which were presented at the 2016 meeting of the Society of Biological Psychiatry, suggest that oxytocin is a promising potential treatment for drug addictions.
Lithium Increases Cortical Thickness in People with Bipolar Disorder
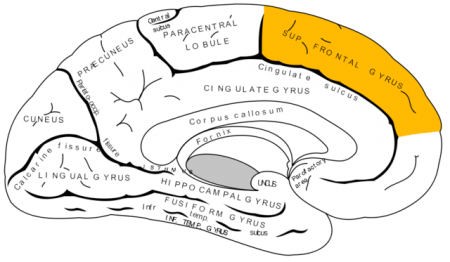 Recent studies have indicated that bipolar disorder is associated with changes to brain volume, including thinning of the cortex. In research presented at the 2016 meeting of the Society of Biological Psychiatry, researcher Noha Abdel Gawad reported that four weeks of lithium treatment increased cortical thickness in the left superior frontal gyrus. This is the third replication of this finding.
Recent studies have indicated that bipolar disorder is associated with changes to brain volume, including thinning of the cortex. In research presented at the 2016 meeting of the Society of Biological Psychiatry, researcher Noha Abdel Gawad reported that four weeks of lithium treatment increased cortical thickness in the left superior frontal gyrus. This is the third replication of this finding.
Other research has established that lithium treatment also increases the volume of the hippocampus in people with bipolar disorder. Together the findings provide strong evidence that lithium treatment protects neurons and can reverse brain changes associated with bipolar disorder.
Lithium Lengthens Telomeres
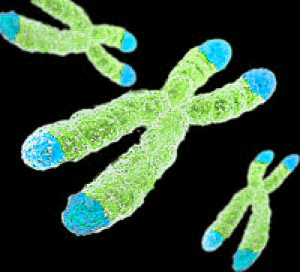 Telomeres are repeated DNA sequences that sit at the end of chromosomes and protect the DNA as it is replicated. Depressive episodes and age can reduce the length of telomeres. Lithium treatment increases telomere length. At the 2016 meeting of the Society of Biological Psychiatry, researcher Martin Schalling reported that the longer a patient takes lithium, the more their telomere length increases.
Telomeres are repeated DNA sequences that sit at the end of chromosomes and protect the DNA as it is replicated. Depressive episodes and age can reduce the length of telomeres. Lithium treatment increases telomere length. At the 2016 meeting of the Society of Biological Psychiatry, researcher Martin Schalling reported that the longer a patient takes lithium, the more their telomere length increases.
According to Schalling, people who respond well to lithium treatment show greater increases in telomere length than those who respond poorly to lithium.
While some cancers are associated with long telomeres, lithium use has not been found to increase cancer risk. In fact, lithium treatment can decrease the risk of certain cancers of the gastrointestinal, respiratory, and endocrine systems.
Azithromycin Antibiotic May Help PANS
PANS is a neuropsychiatric syndrome characterized by the acute onset of obsessive compulsive and other abnormal behaviors, tics, and mood changes that appear in a child following a bacterial or viral infection. PANS refers to any pediatric acute-onset neuropsychiatric syndrome of this type, while PANDAS refers more specifically to such a syndrome that occurs after exposure to streptococcal infections.
New research suggests that treatment with the antibiotic azithromycin can treat PANS. In a study presented at the 2016 meeting of the Society of Biological Psychiatry, Tanya K. Murphy and colleagues found that among 32 children aged 4–14 who showed obsessive compulsive symptoms following an infection, those who were given a 4-week course of azithromycin (10mg/kg of body weight, up to 500 mg/day) saw a 26% drop in symptoms, compared to a 1% drop in symptoms in those who received placebo instead.
At the end of the four weeks, 38.9% of the azithromycin group were classified as much improved or very much improved, while no one in the placebo group achieved this level of improvement. Azithromycin treatment increased the QTc interval (a measure of heart rate) and pulse in the study participants, but did not have any other notable side effects.
PANS is thought to arise from an immune response to infection that goes awry and begins attacking neurons in the brain, particularly in the thalamus. For a more complete review of PANS, see several of our earlier articles about PANS and an excellent review article by researcher Kiki Chang and colleagues in the Journal of Child and Adolescent Psychopharmacology in 2015.
It is important to work up a child suspected of having PANS, as the syndrome does not usually respond to conventional psychiatric treatment and often requires anti-inflammatory drugs (steroids or immunosuppressants), intravenous immunoglobulin (IVIG), plasma exchange, the TNF alpha blocker infliximab, or antibiotics.
Meta-Analysis Shows Anti-Inflammatory Treatments Improve Bipolar Depression
 It has been clear for some time that depression and inflammation are linked. This has led researchers to explore a variety of anti-inflammatory agents to treat depression. A meta-analysis of studies examining anti-inflammatory treatments for bipolar depression was published in the journal Bipolar Disorders in 2016.
It has been clear for some time that depression and inflammation are linked. This has led researchers to explore a variety of anti-inflammatory agents to treat depression. A meta-analysis of studies examining anti-inflammatory treatments for bipolar depression was published in the journal Bipolar Disorders in 2016.
Researcher Joshua D. Rosenblat and colleagues identified eight randomized controlled trials that met their criteria for anti-inflammatory treatments of bipolar disorder. These treatments included nonsteroidal anti-inflammatory drugs (NSAIDs such as ibuprofen and aspirin), omega-3 fatty acids, the antioxidant N-acetylcysteine, and pioglitazone (used to treat diabetes). Overall, the anti-inflammatory treatments had a moderate and statistically significant antidepressant effects. No serious side effects were reported, and the anti-inflammatory treatments did not cause a switch into mania in any of the participants.
The diversity of the anti-inflammatory treatments reviewed in this meta-analysis limit the extent to which it can be interpreted, but it is clear that more research on anti-inflammatory treatments for bipolar depression is needed. An open question is whether patients with particularly elevated levels of inflammatory markers in their blood would respond better to these anti-inflammatory treatments.
Allopregnanolone Injection Eliminated Post-Partum Depression in Four Women
In a small proof-of-concept study, researcher Stephen J. Kanes and colleagues showed that injections of allopregnanolone could nearly eliminate symptoms of post-partum depression.
Allopregnanolone is the main metabolite of the hormone progesterone. Rapid changes in hormone levels following delivery are thought to cause post-partum depression.
In the study, four women with post-partum depression were given injections of SAGE-547, a proprietary solution of allopregnanolone. The dose was adjusted over 12 hours until it approximated prenatal levels of allopregnanolone. This level was maintained for 36 hours, and then the women were weaned off the SAGE-547 over another 12 hours. As soon as the women began injections of SAGE-547, their depression began to improve, and this lasted after they stopped receiving the injections. By 84 hours after beginning treatment, depression scores had improved by 81%.
Kanes and colleagues, who presented this research at the 2016 meeting of the Society of Biological Psychiatry, will follow up this study with placebo-controlled trials of SAGE-547.
Galantamine Did Not Improve Cognitive Deficits in People with Bipolar Disorder
In a recent study by researcher Dan V. Iosifescu and colleagues, the drug galantamine, which is used to treat dementia, did not improve cognitive function in euthymic people with bipolar disorder. The drug had done so in earlier studies. Seventy-two participants with bipolar disorder that was in remission were randomized to receive either a placebo or galantamine extended release for a period of two weeks. Doses of galantamine ranged from 8 to 24 mg/day.
The participants took several tests of attention and memory over the course of the study. After 16 weeks of treatment, those taking galantamine did not show significant improvements in functioning compared to those who received placebo.
This research was presented at the 2016 meeting of the Society of Biological Psychiatry.
Inflammation Linked to Non-Response to Antidepressants
In a symposium on inflammation’s role in psychiatric disorders at the 2016 meeting of the Society of Biological Psychiatry, researcher Carmine Pariante reviewed the considerable literature indicating that major depression is often associated with measures of inflammation. Depression has been linked to elevated blood levels of the inflammatory proteins interleukin-1, interleukin-6, TNF alpha, and c-reactive protein, with about one-third of depressed patients having an elevated level of at least one of these proteins. People with elevated inflammatory markers are also less likely to respond to traditional antidepressants such as selective serotonin reuptake inhibitors (SSRIs).
Pariante reported that in depressed people, interleukin-6 is also elevated in cerebrospinal fluid in addition to blood, suggesting that inflammation in depression extends to the central nervous system. Increased secretion of interleukin-6 has been linked to depressive behaviors in mice exposed to stress.
There is some hope that anti-inflammatory treatments can help patients who do not respond to traditional antidepressant treatment. Some anti-inflammatory medications that may eventually be used to treat depression with inflammation include the COX-1 inhibitor aspirin, the COX-2 inhibitor celecoxib (Celebrex), or the antibiotic minocycline. Each of these approaches gained some support in preliminary clinical trials, but it has not yet been established that these anti-inflammatory treatments produce a better response in people with elevated inflammatory markers.
Methylene Blue May Help Bipolar Depression
 We have previously reported on the research by Martin Alda and colleagues that the chemical compound methylene blue had positive effects in patients with bipolar depression. The research was published in the British Journal of Psychiatry in 2016.
We have previously reported on the research by Martin Alda and colleagues that the chemical compound methylene blue had positive effects in patients with bipolar depression. The research was published in the British Journal of Psychiatry in 2016.
Now a new article by Ashley M. Feen and colleagues in the Journal of Neurotrauma reports that methylene blue has an antidepressant-like effect in mice with traumatic brain injury (TBI). Methylene blue reduced inflammation and microglia activation in the animals. Methylene blue reduced levels of the pro-inflammatory cytokine Il-1b and increased levels of the anti-inflammatory cytokine Il-10.
These findings are of particular interest as many patients with classical depression (and no brain injury) have abnormal levels of these inflammatory markers. It remains to be seen whether methylene blue is more helpful in those patients with elevated inflammatory markers and if levels of the markers can predict treatment response or not.
Methylene blue causes urine to turn blue, so low doses of the compound are used as a placebo. Alda and colleagues reported that the active dose 195mg reduced depression and anxiety significantly more than the placebo dose (15mg) in a 13-week crossover study. In that study, methylene blue was added to lamotrigine which had not had a complete enough effect.
In a 1986 study by G.J. Naylor and colleagues in the journal Biological Psychiatry, patients were treated with either 15mg/day or 300mg/day of methylene blue for one year and crossed over to the other dose in the second year. Participants had significantly less depression during the year of taking the active 300mg/day dose.
The FDA has issued a warning about the danger of a serotonin syndrome if methylene blue is combined with serotonin active agents (presumably because it inhibits MAO-A). Symptoms of the serotonin syndrome can include lethargy, confusion, delirium, agitation, aggression, decreased alertness, and coma. Neurological symptoms, such as jerky muscle contractions, loss of speech, muscle tension, and seizures; or autonomic symptoms, such as fever and elevated blood pressure, are also common. Patients should call their doctor if they are taking a serotonergic psychiatric medication and develop any of the above symptoms.






