Diabetes Complicates Bipolar Disorder
People with bipolar disorder are three times more likely than the general population to develop type 2 diabetes. Type 2 diabetes typically occurs in adulthood and is associated with insulin resistance, as opposed to type 1, which is usually diagnosed in childhood and is associated with insulin deficiency.
In a talk at the 2015 meeting of the Society of Biological Psychiatry, researcher Tomas Hajek reported that in a large group of bipolar patients, 13% reported a history of type 2 diabetes, 21% were diagnosed with type 2 diabetes upon laboratory evaluation, and 32.2% had pre-diabetes without realizing it. Thus, about half of these patients with bipolar disorder were either affected by diabetes or at risk for it, many without knowing it.
The Bad News
Diabetes complicates the course of bipolar illness. Type 2 diabetes is associated with poorer response to treatment, atrophy of the hippocampus, cognitive impairment, and higher rates of conversion from mild cognitive impairment to full-blown dementia.
The main effect of type 2 diabetes is insulin resistance. The body produces enough insulin, but insulin’s effects at its receptors are impaired. Diabetes also causes deficits in growth factors, increases in the enzyme GSK3B, decreases in mitochondria and brain-derived neurotrophic factor (BDNF, which protects neurons), and glucose toxicity.
Recent research by Hajek and colleagues shows that diabetes has several other detrimental effects on the brain in bipolar disorder. On magnetic resonance spectroscopy (MRS) scans, people with type 2 diabetes had lower levels of NAA, a marker of neuronal integrity, in the prefrontal cortex. This can indicate impaired functioning. People with type 2 diabetes also had lower levels of creatine, indicating impaired energy metabolism. In addition, hippocampal volume decreases with aging, and type 2 diabetes accelerated this age-related decline.
Some of diabetes’ effects on the brain are mediated by other health factors, including obesity, cerebral blood vessel disease (which affects white matter integrity), and side effects from medications.
What You Can Do
Start early with a good diet and exercise, and have regular checkups with a doctor, who can tell you if you have diabetes or are at risk for it. If so, start long-term preventative treatment with the most effective and easy-to-tolerate medications, and periodically have your fasting blood sugar tested. If these tests are abnormal, have your hemoglobin A1c (HbA1c) checked. This is a measure of good glucose control, and should be under 6. If it creeps upward toward 6 (a sign of pre-diabetes), the drug metformin may be able to prevent the onset of type 2 diabetes. If you have type 2 diabetes, there are several types of effective medications that can minimize its effects.
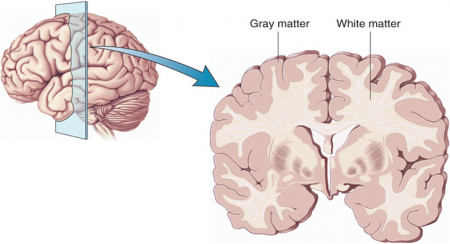
Atypical Antipsychotics May Slow Loss of Gray Matter in Schizophrenia
Progressive losses in gray matter have been observed in the cortex of people with schizophrenia, and those at high risk for the illness. In the past, studies have shown that the amount of antipsychotics a patient is exposed to is correlated with the extent of their deficits in gray matter, suggesting that antipsychotic treatment could exacerbate gray matter loss.
A new meta-analysis by Antotonio Vita and colleagues in the journal Biological Psychiatry shows that first-generation antipsychotics were associated with greater losses in gray matter compared with atypical antipsychotics, which seemed to slow the loss of gray matter.
The meta-analysis analyzed data from 18 longitudinal studies comparing a total of 1155 patients with schizophrenia to 911 healthy control participants. Magnetic resonance imaging (MRI) scans showed that over time, patients with schizophrenia lost more cortical gray matter volume. The patients’ cumulative intake of any kind of antipsychotic between MRI scans was associated with gray matter losses. But when Vita and colleagues drilled down to find differences between patients taking first-generation antipsychotics and those taking second-generation atypical antipsychotics, they found that patients with higher average daily intake of first-generation antipsychotics had greater losses in gray matter, while patients with higher average daily intake of atypical antipsychotics had less progressive losses in gray matter.
This study is the first to compare the effects of first-generation antipsychotics, which were developed in the 1960s, with those of atypical antipsychotics, which came into frequent use in the late 1980s, on cortical gray matter loss in schizophrenia. While first-generation antipsychotics are associated with the side effect of tardive dyskinesia, involuntary movements of the face and jaw, atypical antipsychotics are most commonly associated with weight gain.
Three studies have randomly assigned patients with schizophrenia to receive either first-generation or atypical antipsychotics. In these studies as well, second-generation antipsychotics were associated with smaller losses in gray matter.
The authors speculate that either second-generation antipsychotics may have neuroprotective effects, or first-generation antipsychotics may have neurotoxic effects. They also suggest that first-generation antipsychotics may not have the capacity to interfere with the natural progression of schizophrenia in terms of gray matter losses.
Future studies may investigate differences between specific antipsychotic medications’ effects on gray matter volume. Vita and colleagues reported that in the analysis, the atypical antipsychotic clozapine was associated with the least loss of gray matter of any medication in the included studies.
Editor’s Note: This study is important because it adds to findings questioning the conclusions of a large National Institute of Mental Health–sponsored study known as CATIE and a meta-analysis by John Geddes published in the journal BMJ in 2000, in which he wrote that “There is no clear evidence that atypical antipsychotics are more effective or better tolerated than conventional (first generation) antipsychotics.” Read more
Nutritional Supplement Minimizes Stress After a Natural Disaster
Certain nutritional supplements may help people cope following natural disasters. Following a 7.1 magnitude earthquake in Christchurch, New Zealand, in 2010, researchers there who were working on a clinical trial of a broad spectrum mineral and vitamin formula for ADHD realized that they could compare participants who had been taking the nutritional supplements at the time of the earthquake with those who had either already completed the trial or had not yet begun it. Two weeks after the quake, those who had been taking the multivitamin at the time of the quake were less anxious and stressed than those who hadn’t been taking the formula.
When another large earthquake struck five months later, the researchers implemented a randomized trial comparing two doses of the same broad spectrum supplement with a B Complex vitamin formula that had previously shown efficacy for stress and anxiety. Those participants taking any supplement showed fewer symptoms of post-traumatic stress disorder (PTSD) a month after the second quake compared to controls, and those taking the higher dose of the broad spectrum formula had greater improvements in mood and anxiety than those taking the B Complex supplement.
More recently, in Alberta, Canada, flooding forced many people from their homes. Researchers there who were studying the effects of micronutrients on stress and anxiety realized they had the opportunity to replicate the research from New Zealand in a different type of environmental disaster.
Researcher Bonnie J. Kaplan and colleagues recruited adults who had been affected by the flood, and randomized the participants to receive different types of supplements: vitamin D (1 pill/day); a B complex vitamin containing B6, B12, and several other nutrients (1 pill/day); or a broad spectrum supplement containing 24 vitamins and minerals and several botanical extracts (4 pills/day). No placebo was used—it was considered unethical to deny participants access to a potentially helpful treatment.
In a 2015 article in the journal Psychiatry Research, the Alberta team reported that while all of the nutrient supplements minimized stress after the flood, patients randomized to the B complex vitamin or the broad spectrum formula had less stress and anxiety following the flood than those randomized to vitamin D.
We have previously described a broad spectrum vitamin preparation called EMPowerplus, used by psychiatrist Charles Popper and psychologist Mary A. Fristad to treat children with treatment-resistant bipolar disorder. This may be the same formula used in the Alberta study. We await larger trials of this preparation in children with bipolar disorder.
Preliminary Evidence That Anti-Inflammatory Celecoxib Helps in Bipolar Depression
A study currently in progress indicates that the anti-inflammatory COX-2 inhibitor celecoxib (better known as the arthritis treatment Celebrex) may aid in the treatment of bipolar depression. In a panel session on inflammation at the 2015 meeting of the Society of Biological Psychiatry, researcher Angelos Halaris reported results from the first 26 participants.
Participants were taking mood stabilizers for bipolar disorder and became depressed. They received either 20mg/day of the selective serotonin reuptake inhibitor antidepressant escitalopram (Lexapro) plus either 200mg twice a day of celecoxib or placebo for a total of eight weeks. Those participants who received celecoxib showed greater and more rapid reductions in depression symptoms than those who received placebo.
The study will continue, and Halaris and colleagues will also observe whether measures of inflammation in patients’ blood are correlated with the patients’ responsiveness to the combined treatment with escitalopram and celecoxib.
Low Oxytocin Linked to Depression in Moms
At a panel at the 2015 meeting of the Society of Biological Psychiatry, researcher Andrea Gonzales described her team’s study of mechanisms related to postpartum depression and the bonding hormone oxytocin. In the study of 26 women at eight months postpartum, the team examined whether there were connections between a mother’s levels of oxytocin at baseline and after interacting with her child, her mood symptoms, and whether she was mistreated in childhood.
Those women who scored low on a history of maltreatment in childhood had bigger increases in oxytocin in their blood and saliva after interacting with their children. Those with high trauma scores but low levels of depression also saw big boosts in oxytocin after seeing their children. Those women who had both a history of trauma in childhood and current depressive symptoms did not get as big a boost of oxytocin after interacting with their children.
Gonzales and colleagues concluded that postpartum depression is linked to dysregulation of oxytocin levels, and that a history of trauma in the mother’s childhood can make this worse.
The researchers hope that these findings may make it easier to identify which women are at risk for postpartum depression, and that they may point to possible treatments in the future.
Postpartum depression is a problem for about 13% of mothers in the year after they give birth, and mother-child bonding may be disturbed if a mother is depressed. One way to foster better bonding between a depressed mother and her newborn is to use video feedback. A mother views video of herself interacting with her child while a trained professional helps her identify opportunities for greater physical contact.
Omega-3 Fatty Acids Prevent Conversion to Psychosis
 A new long-term study of omega-3 polyunsaturated fatty acids for psychosis prevention shows that almost seven years after a 3-month stint of receiving these dietary supplements daily, adolescents and young adults at high risk for psychosis showed fewer symptoms of conversion to full-blown psychosis than those who received placebo during the same period.
A new long-term study of omega-3 polyunsaturated fatty acids for psychosis prevention shows that almost seven years after a 3-month stint of receiving these dietary supplements daily, adolescents and young adults at high risk for psychosis showed fewer symptoms of conversion to full-blown psychosis than those who received placebo during the same period.
The research team, led by Paul Amminger, originally found that among 81 youth (mean age 16.5) at high risk of developing psychosis due to their family histories, the 41 who received 12 weeks of daily supplementation with 700mg of eicosapentaenoic acid (EPA) omega-3s and 480 mg of docosahexaenoic acid (DHA) omega-3s showed reduced likelihood of conversion to psychosis one year later than the 40 who received placebo.
The team followed up an average of 6.7 years later with 71 of the original 81 participants. Among those who had received the omega-3 intervention, 9.8% had developed psychosis. Among the placebo group, 40% had developed psychosis, and they had done so earlier.
In addition, the omega-3 participants were better functioning, they had required less antipsychotic medication, and they had lower rates of any psychiatric disorder than the placebo group.
Amminger wrote in the journal Nature Communications, “Unlike antipsychotics, fish oil tablets have no side effects and arent’s stigmatizing to patients.”
Editor’s Note: Because of their lack of side effects, a good case can be made for omega-3 fatty acids for patients at high risk for psychosis. The novel thing about this study is that short-term treatment with omega-3 fatty acids had preventive effects almost 7 years later.
Blood and Now Brain Inflammation Linked to Depression
 There is growing evidence of a link between inflammation of depression. At the 2015 meeting of the Society of Biological Psychiatry, researcher Jeff Meyer summarized past studies on inflammatory markers. These are measurements, for example of certain proteins in the blood, that indicate the presence of inflammation in the body.
There is growing evidence of a link between inflammation of depression. At the 2015 meeting of the Society of Biological Psychiatry, researcher Jeff Meyer summarized past studies on inflammatory markers. These are measurements, for example of certain proteins in the blood, that indicate the presence of inflammation in the body.
Common inflammatory markers that have been linked to depression include IL-6, TNF-alpha, and c-reactive protein. At the meeting, Meyer reviewed the findings on each of these. Twelve studies showed that IL-6 levels are elevated in the blood of patients with depression. Four studies had non-significant results of link between IL-6 and depression, and Meyer found no studies indicating that IL-6 levels were lower in those with depression. Similarly, for TNF-alpha, Meyer found 11 studies linking elevated TNF-alpha with depression, four with non-significant results, and none showing a negative relationship between TNF-alpha and depression. For c-reactive protein, six studies showed that c-reactive protein was elevated in people with depression, six had non-significant results, and none indicated that c-reactive protein was lower in depressed patients.
Most studies that have linked inflammation to depression have done so by measuring inflammatory markers in the blood. It is more difficult to measure inflammation in the brain of living people, but Meyer has taken advantage of new developments in positron emission tomography (PET) scans to measure translocator protein binding, which illustrates when microglia are activated. Microglial activation is a sign of inflammation. Translocator protein binding was elevated by about 30% in the prefrontal cortex, anterior cingulate cortex, and insula in study participants who showed symptoms of a major depressive episode compared to healthy control participants. The implication is that the depressed people with elevated translocator protein binding have more brain inflammation, probably via microglial activation.
The antibiotic minocycline reduces microglial activation. It would be interesting to see if minocycline might have antidepressant effects in people with depression symptoms and elevated translocator protein binding.
Autopsy Studies Show Brain Inflammation in Unipolar Depression, Bipolar Disorder, and Suicide
 Depression and bipolar disorder have been linked to high levels of inflammatory proteins in the blood (namely CRP, IL-1, IL-6, and TNF-alpha), but the relationship between these illnesses and inflammation in the brain has not been well-characterized.
Depression and bipolar disorder have been linked to high levels of inflammatory proteins in the blood (namely CRP, IL-1, IL-6, and TNF-alpha), but the relationship between these illnesses and inflammation in the brain has not been well-characterized.
At the 2015 meeting of the Society for Biological Psychiatry, researcher Ghanshyan Pandey discussed findings from autopsy studies of people who died with a diagnosis of unipolar depression or bipolar disorder, and teens who died of suicide. The studies compare data from these ill people with those of controls who are matched for demographic characteristics.
Pandey found that the brains of those who died of unipolar depression and bipolar disorder showed more signs of inflammation compared to the controls. This included elevated levels of the inflammatory proteins IL-1B, IL-6, and TNF-alpha, in addition to elevated levels of the mRNA that leads to their production. Pandey also found that those with depression and bipolar disorder had higher levels of mRNA for the receptors to which TNF-alpha and other inflammatory proteins attach themselves.
Pandey performed similar autopsy studies of teens who died of suicide, the second leading cause of death for this age group, compared to teens who died of other causes. There were more signs of inflammation in the prefrontal cortices of teens who died of suicide. These included mRNA and proteins for IL-1B and TNF-alpha, and IL-6 proteins. In contrast to the ill adults, the teens who died of suicide had lower levels of the receptors for inflammatory proteins than controls. Another type of receptor known as toll-like receptors was higher in the ill teens, particularly the mRNA and proteins TLR3 and TLR4.
US Lags Behind Canada in Access to RTMS
At the 2015 meeting of the Transcranial Magnetic Stimulation Society, Linda Carpenter, an American researcher who specializes in repeated transcranial magnetic stimulation (rTMS), a method of treating depression by using a magnetic coil placed near the scalp to stimulate neurons, compared notes with Jeff Daskalakis, a Canadian researcher who also studies rTMS.
Carpenter described the limited approval rTMS enjoys in the US. RTMS has been approved by the Federal Drug Administration for the treatment of unipolar depression under very limited parameters (only at a frequency of 10Hz). RTMS has limited availability in the US, and many healthcare companies do not cover it. Providers face scrutiny of study recruitment practices and recordkeeping by insurers and the Joint Commission (formerly the Joint Commission of Accreditation of Healthcare Organizations), which assesses healthcare quality.
In contrast, Daskalakis and his Canadian colleagues can and do use rTMS to treat a broader range of illnesses including bipolar disorder. In Canada rTMS is used to treat unipolar depression, schizophrenia, post-traumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD), and clinicians can adjust the parameters to treat adolescents and the elderly.
The situation in the US is unfair. Because rTMS has not been approved for the treatment of bipolar disorder, Carpenter and other clinicians in the US are unable to treat bipolar depression even though a wide range of experts and published studies report that rTMS is as effective (or possibly even more so) for patients with bipolar depression than for those with unipolar depression.
Few treatments are available for bipolar depression. The discrepancy is even sadder when one considers that there are already more than 20 FDA-approved antidepressants that can be used to treat unipolar depression, but only three approved medications for bipolar depression. Bipolar depression is an orphan illness, which lacks a powerful voice advocating for more treatment research about optimal therapeutic strategies. Read more
RTMS in the Elderly and After ECT
At the 2015 meeting of the Society of Biological Psychiatry in May, researcher Daniel Blumberger reported to this editor (Robert M. Post) that he has found repeated transcranial magnetic stimulation (rTMS) to be effective for depression in late life. Blumberger noted that it may be necessary to use higher intensity stimulation (i.e. at 120% of motor threshold instead of the usual 110% of motor threshold) in the elderly in order to overcome the gap between the skull and the brain, which can grow with age due to brain atrophy.
Blumberger has also successfully used rTMS as a followup treatment to a successful course of electroconvulsive therapy (ECT), administering rTMS twice a week for up to 66 treatments in a given patient in order to maintain remission of their depression.