Trace lithium levels in drinking water reduce the risk of dementia: a systematic review
International Journal of Bipolar Disorders volume 12, Article number: 32 (2024)
The sample size varied in the studies from 37,597 to 35,000,000. Lithium levels ranged from 0.002 to 0.027 (mg/L).
“We systematically reviewed five available studies, which reported associations between trace-Li in water and incidence [of] or mortality from dementia. Association between trace-Li levels and a lower risk or mortality from dementia were observed at concentrations of Li in drinking water as low as 0.002 mg/L and 0.056 mg/L. Meanwhile, levels below 0.002 mg/L did not elicit this effect. Although three of the five studies found dementia protective properties of Li in both sexes, a single study including lower Li levels (0.002 mg/l) found such association only in women.
Conclusion
The reviewed evidence shows that trace-Li levels in the water are sufficient to lower the incidence or mortality from dementia. Considering the lack of options for the prevention or treatment of dementia, we should not ignore these findings. Future trials of Li should focus on long term use of low or even micro doses of Li in the prevention or treatment of dementia.
Vortioxetine Improves Cognition, Major Depression in Early Dementia
Vortioxetine (5mg then 10mg) significantly improves depressive symptoms, cognitive performance, functioning, and quality of life at 12 weeks in patients with both major depressive disorder (MDD) and early-stage dementia. In addition to blocking 5HT (serotonin) reuptake vortioxetine antagonizes 5 serotonin receptors with 5HT3 and 5HT7 likely accounting for the positive effects on processing speed and cognitive functioning.
Antihypertensives That Stimulate vs Inhibit Type 2 and 4 Angiotensin II Receptors Decrease Dementia
Marcum et al in JAMA New Open (2023) found that in “57,773 Medicare beneficiaries, initiation of antihypertensive medications that stimulate vs inhibit type 2 and 4 angiotensin II receptors was associated with a statistically significant 16% lower risk of incident dementia, over a median of 6.9 years of follow-up.”
“Angiotensin II receptor type 2 and 4–stimulating antihypertensive medications (hereafter, stimulating medications) included: Angiotensin II receptor type 1 blockers, dihydropyridine calcium channel blockers, and thiazide diuretics.
Angiotensin II receptor type 2 and 4–inhibiting antihypertensive medications (hereafter, inhibiting medications) included: angiotensin-converting enzyme (ACE) inhibitors, ?-blockers, and nondihydropyridine calcium channel blockers.”
Editors Note: If you have hypertension and are at risk for cognitive decline, know that your choice of effective antihypertensive drugs can lead to better cognitive outcomes. Drugs that stimulate the angiotensin II receptor type 2 and 4 help prevent dementia. These drugs include:
ARB type 1, dihydropyridine calcium channel blockers, and thiazide diuretics. (Good guys)
Those that inhibit Angiotensin II receptors types 2 and 4 do not prevent dementia. These drugs include:
ACE inhibitors, beta blockers, and non-dihydropyridine calcium channel blockers. (Bad guys)
Talk with your doc about drugs equally for blood pressure control but those that also have benefits for ultimate preservation of cognition.
Transcranial Near-Infrared Light May Treat Brain Injury and Neurodegeneration
At the 2021 meeting of the Society of Biological Psychiatry, there was a symposium on treatment with near-infrared light chaired by researchers Paolo Cassano and Dan Iosifescu. The treatment is known as transcranial photobiomodulation (PBM) with near-infrared light. A device worn on the head delivers infrared light that penetrates the cerebral cortex and can modulate cortical excitability. It has a variety of effects including promoting neuroplasticity, improving oxygenation, and decreasing inflammation and oxidative stress.
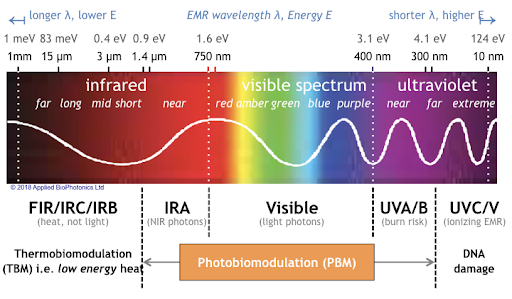
A number of studies exploring the possibility that PBM could be used as a clinical treatment for conditions such as depression, brain injury, or dementia were presented at the symposium.
Researcher Lorelei Tucker discussed promising findings from an animal model in which rats with stroke or brain injuries showed improvement after being treated with PBM.
Cassano discussed studies aimed at refining which brain areas should be targeted with PBM and how much light should be delivered in order to improve depression. In double-blind, sham-controlled studies in people with major depression, targeting the dorsolateral prefrontal cortex with PBM improved their symptoms.
Researcher Benjamin Vakoc discussed a study of low-level light therapy (LLLT) using near-infrared light compared to a sham procedure in 68 people with moderate traumatic brain injury. The researchers used magnetic resonance imaging (MRI) to assess changes in white matter in the brain over time in people recovering from an acute brain injury. Patterns of changes in white matter were different for those who received LLLT compared to those who received the sham procedure.
Researcher Linda Chao described a very small study to determine whether PBM could improve symptoms of dementia. Four patients received typical dementia care while four others underwent home treatments with the commercially available Vielight Neuro Gamma device, which delivers PBM via both the scalp and an insert in the nose. After 12 weeks, the PBM group showed improvements in cognition and brain connectivity.

Editor’s Note: We will be watching the literature to follow advances in this promising novel method of neuromodulation.
Pimavanserin Prevents Relapse in Patients with Dementia-Related Psychosis

At a recent scientific meeting, Erin Foff of Acadia Pharmaceuticals Inc. described a study of pimavanserin (a selective serotonin inverse agonist/antagonist at 5-HT2A receptors) in dementia-related psychosis. Pimavanserin is currently approved in the United States for the treatment of hallucinations and delusions associated with Parkinson’s disease (PD). There is currently no Food and Drug Administration–approved treatment for dementia-related psychosis.
Enrolled patients had moderate-to-severe psychosis associated with Alzheimer’s disease, Parkinson’s, dementia with Lewy bodies, vascular dementia, or frontotemporal dementia. After a 12-week open label phase with flexible dosing and a target dosage of 34mg/day, 217 of the participants with a good response to pimavanserin were then randomized to continue pimavanserin or switch to placebo. The study was stopped early when a prespecified interim analysis revealed that pimavanserin was clearly superior to placebo. There was a more than 2.8-fold reduction in risk of relapse with pimavanserin compared to placebo in the double-blind period. Those on higher doses of 34mg/day showed a more than 3.4-fold reduced risk of relapse. Acadia will seek FDA approval for pimavanserin for the treatment of dementia-related psychosis.
No Association of Benzodiazepines, Z Drugs and Other Anxiolytics with Dementia

Benzodiazepines, so-called Z-drugs (such as zolpidem, zopiclone, and zaleplon), and other anxiolytics are commonly prescribed drugs that have some cognitive side effects. For this reason, there has been concern that the drugs may increase risk of dementia, and small studies had suggested that this might be the case. However, a new large study found no subsequent dementia risk after taking these drugs.
In a 2020 article in the American Journal of Psychiatry, researchers Merete Osler and Martin Balslev Jørgensen described a cohort and nested case-control study of 235,465 adult patients in Denmark in which they found no association of benzodiazepines, Z-drugs, or other anxiolytics with a subsequent diagnosis of dementia. Participants were patients over the age of 20 who were hospitalized for an affective disorder. Of these, 75.9% had been prescribed one of the drugs in question, and 4.2% went on to be diagnosed with dementia.
While participants in this study who had the lowest use of benzodiazepines or Z drugs showed a minimal increased risk of dementia compared to those who took none of these drugs, those who had the highest use of benzodiazepines and Z drugs actually had the lowest incidence of dementia in the study.
The previous studies may have been “confounded by indication” meaning they did not take the underlying psychiatric condition for which the drugs were prescribed into account.
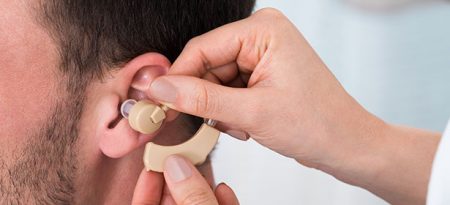
Hearing Aids May Lessen Cognitive Decline, Memory Loss
A 2018 article by researcher Asri Maharani and colleagues in the Journal of the American Geriatrics Society reports that using a hearing aid was associated with better scores on a test of episodic memory, and that declines in episodic memory slowed after participants began using hearing aids.
The study included 2,040 adults aged 50 years and up. Maharani and colleagues used data from the Health and Retirement Study, which measured participants’ cognitive functioning every two years for 18 years. Participants were asked to recall 10 words both immediately and after some delay.
The authors suggested that improving access to hearing aids earlier in the course of hearing impairment might help to stem the rise of dementia.
Naturally Occurring Lithium in Texas Drinking Water Reduced Alzheimer’s Mortality Rates
Several studies have found that trace levels of lithium that naturally occur in the drinking water of certain regions are associated with reductions in dementia compared to regions with less lithium in the water. The latest such study found that higher trace levels of lithium in certain Texas counties were associated with less mortality from Alzheimer’s disease compared to Texas counties with lower levels of lithium in the water.
The research by Val Andrew Fajardo and colleagues was published in the Journal of Alzheimer’s Disease in 2017. Fajardo’s team obtained 6,180 water samples from 234 of Texas’ 254 counties. They also calculated that there was an increase in the Alzheimer’s mortality rate from the period 2000–2006 to the period 2009–2015. However, regions with higher trace levels of lithium were negatively correlated with this increase, suggesting that the lithium in the water had a protective effect on people in those counties.
The researchers controlled for gender, race, education, rural living, and air pollution. Physical inactivity, obesity, and type 2 diabetes seemed to be confounding factors. Obesity and type 2 diabetes were positively correlated with Alzheimer’s mortality and negatively correlated with lithium levels in drinking water, meaning that it is possible that lithium also protects against these conditions.
Lithium in Drinking Water May Reduce Dementia
New research suggests that higher trace levels of lithium in drinking water can reduce dementia rates in the general population. In a 2017 article in the Archives of General Psychiatry, researcher Lars Kessing and colleagues compared data on 73,731 patients in Denmark with a diagnosis of dementia to 733,653 control participants without this diagnosis between the years 1970 and 2013. They were able to match the data to recorded levels of trace lithium in the drinking water in participants’ municipalities of residence.
Lithium levels in the water ranged from 0.6 micrograms per liter to 30.7 micrograms per liter in 151 different locations throughout Denmark. Compared to those exposed to 2.0 to 5.0 micrograms of lithium per liter of water, those exposed to more than 15.0 micrograms per liter had a lower incidence rate of dementia. However, those exposed to 5.1 to 10.0 micrograms per liter had a higher incidence of dementia. The same relationship was also found between lithium exposure levels and both Alzheimer’s disease and vascular dementia.
The lithium levels in the water were approximately 10,000 to 300 times lower than typical clinical doses (typically 900–1500mg/day, which produce concentrations ranging from 0.6 to 1.2 meq/L in patients’ blood). The minute exposures to lithium in the drinking water occurred over decades in the Danish study, and suggest that there may be long-term positive effects to chronic lifetime exposure to very low lithium levels.
These data follow others regarding exposure to trace lithium. In 2011, researcher Orestes V. Forlenza and colleagues reported in the British Journal of Psychiatry that low dose lithium (150–600mg/day) over a period of one year decreased the progression of mild cognitive impairment compared to placebo, while researcher Marielza Andrade Nunes and colleagues reported in the journal Current Alzheimer’s Research in 2013 that an even smaller dose (0.3mg/day) over a period of 15 months slowed the progression of Alzheimer’s dementia. Thus, low or microscopic doses consumed over long periods could slow cognitive deterioration.
Caffeine One of Several Compounds That May Protect Against Dementia
A 2017 article by Yousuf O. Ali and colleagues in the journal Scientific Reports finds that 24 different compounds may boost a brain enzyme that protects against dementia. The enzyme, NMNAT2, protects neurons from stress and combats misfolded proteins called tau that form plaques in the brain as people age.
Ali and colleagues screened 1280 compounds to identify those that might increase NMNAT2 production. Twenty-four of these looked promising, including caffeine and rolipram, an “orphaned drug” once studied as an antidepressant but discontinued in the 1990s. Others with weaker effects on NMNAT2 production included the atypical antipsychotic ziprasidone, cantharidin (a wart-removing substance secreted by blister beetles), fungal metabolite wortmannin, and retinoic acid, a vitamin A derivative. Thirteen of the compounds tested decreased NMNAT2 production.
The researchers followed up the caffeine finding by testing caffeine in mice genetically engineered to produce less NMNAT2. The caffeine administration normalized NMNAT2 production levels in these mice.
Senior researcher Hui-Chen Lu hopes this research will lead to the development of new drugs that can create a chemical blockade against the effects of neurodegenerative illnesses.