New Medication for PTSD Approved
The double-blind, phase 3 trial included 416 adults aged 18-65 years with a DSM-5 diagnosis of PTSD and symptoms for at least 6 months prior to screening.
Patients underwent a 1-week placebo-run in period followed by randomization to daily oral brexpiprazole 2-3 mg plus sertraline 150 mg or daily sertraline 150 mg plus placebo for 11 weeks.
“Epigenetic Changes After Trauma May Be Adaptive, Contribute to Resilience”
Originally From Psychiatric News Update
In recent years, research throughout the scientific and medical community has suggested a link between trauma and epigenetic changes, chemical modifications that affect gene activity without actually changing the gene’s DNA sequence. The assumption has been that epigenetic changes in the context of trauma are inherently bad, a form of damage that gets passed from generation to generation. But according to Rachel Yehuda, Ph.D., Endowed Professor of Psychiatry and Neuroscience of Trauma at the Icahn School of Medicine at Mount Sinai, these changes may also be adaptations that promote resilience.
“Sometimes the biological changes in response to trauma or intergenerational trauma are there to help deal with the problem of trauma, not compound its effects,” Yehuda said. “The survival advantage of this form of intergenerational transmission depends in large part on the environment encountered by the offspring themselves.”
Yehuda described this phenomenon as a paradox.
“Parental or ancestral trauma may heighten vulnerability to mental health challenges, but epigenetic adaptations may simultaneously facilitate coping mechanisms,” she said. “Trauma increases susceptibility for psychological distress, but also produces adaptations that help us cope with them.”
Yehuda described research she and her colleagues have conducted to tease out how trauma in parents can affect offspring in the context of the biology of posttraumatic stress disorder (PTSD) in Holocaust survivors and their children. As the research unfolded, Yehuda and colleagues found that survivors’ adult children were more likely to have mood disorders, anxiety disorders, and PTSD than Jewish people whose parents did not directly experience the Holocaust. This was especially true of children of Holocaust survivors who had PTSD. The researchers also found that many children of Holocaust survivors had low levels of the stress hormone cortisol, particularly if their parents had PTSD.
Yehuda and colleagues then conducted a series of studies that looked at the role of glucocorticoid receptors — the proteins to which cortisol must bind to exert its effects — and found evidence that these receptors were more sensitive in people with PTSD.
“In practical terms this means that even though someone with PTSD might have lower circulating levels of cortisol in their blood, their cells might react more strongly to the cortisol that is present,” Yehuda said.
Yehuda said that epigenetics provided further insight on the relationship between hypersensitive glucocorticoid receptors, cortisol, and PTSD. She explained the potential role of methylation, which is a chemical reaction in the body in which a small molecule called a methyl group gets added to DNA or DNA-associated proteins.
“Increased methylation generally impedes RNA transcription, whereas less methylation enhances gene expression,” Yehuda said.
In 2015, Yehuda and colleagues conducted a study involving combat veterans who had PTSD and found lower methylation on an important region on the participants’ glucocorticoid receptor gene. The changes were associated with cortisol and glucocorticoid receptor sensitivity in the study participants, suggesting a potential epigenetic explanation for the association between the trauma of combat and PTSD.
Yehuda said that stress-related epigenetic changes may be reversible. For example, one of the studies conducted by her team revealed that combat veterans with PTSD who benefited from cognitive-behavioral psychotherapy showed treatment-induced changes in the methylation of a gene that regulates glucocorticoid receptor sensitivity. Yehuda said that this finding confirmed that healing is also reflected in epigenetic change.
“That we can transform to meet environmental challenge is a superpower. That is resilience,” Yehuda said.” ?
Yehuda then went on to describe the striking and lasting effects of the psychedelics psilocybin and MDMA in trauma and in helping patients confront their fears in a positive and hopeful fashion. These agents which are given with intensive psychotherapeutic support are not yet FDA approved, but preliminary data suggest that they can have dramatic therapeutic effects in trauma and depression. They can help patients change their attitudes to themselves and the world.
Cannabis and Cannabinoids Don’t Work for Pain or Posttraumatic Stress Disorder
Aaron S. Wolfgang, MD and Charles W. Hoge, MD reviewed data on cannabis in JAMA Psychiatry and found that there were big placebo effects and no evidence for effectiveness of cannabis in military personal.
This negative data, along will all the liability of cannabis potentially causing or triggering psychosis, bipolar disorder, and schizophrenia (as well as possibly contributing to cognitive dysfunction, worsening anxiety and depression in patients with mood disorders) makes the use of pot for medical purposes an entirely foolhardy proposition, as well as a waste of money.
Legalization of pot has helped people avoid jail but precipitated a rash of use and over use.
So the bottom line from this editor is: Get Your Priorities Straight. Cannabis and Cannabinoids Don’t Work for Pain or Posttraumatic Stress Disorder and they Worsen Most Everything Else. Save your Money and Do Something Nice for Yourself and Others Instead.
MDMA Superior to Placebo in Treatment of Severe PTSD

An article by researcher Jennifer M. Mitchell and colleagues published in the journal Nature in 2021 reported that MDMA was more effective than placebo at treating severe post-traumatic stress disorder in an 18-week phase 3 clinical trial.
The article reported on a randomized, double-blind, placebo-controlled, multi-site trial that took place in the US, Canada, and Israel. Participants in the trial had severe PTSD, and in some cases also had dissociation, depression, a history of alcohol or substance use disorders, or childhood trauma. In the trial, 91 participants were required to stop any current psychiatric medications for a “washout” period, and then were randomized to either a group that received 12 therapy sessions plus placebo or a group that received 12 therapy sessions and 3 doses of MDMA, which were administered in a clinical setting.
Two months after the last therapy session, participants who had received MDMA during the trial showed greater reductions in PTSD symptoms and less functional impairment than those who were assigned to the placebo group during the trial. At the endpoint of the study, 67% of participants in the MDMA group no longer met the criteria for a PTSD diagnosis, compared to 32% of the placebo group.
Side effects that were more prevalent among the MDMA group were typically transient and mild to moderate in severity. These included muscle tightness, decreased appetite, nausea, sweating, feeling cold, and increased blood pressure and heartrate. The MDMA group did not show any increases in drug abuse or suicidality compared with the placebo group, and MDMA had similar effects among those participants with and without comorbid conditions. The authors concluded that MDMA was highly efficacious, safe, and well-tolerated as a treatment for PTSD and urged that further study of the treatment be done without delay.
Currently, US Food and Drug Administration (FDA)–approved treatments for PTSD include the selective serotonin reuptake inhibitors (SSRIs) sertraline and paroxetine, but approximately half of PTSD patients do not respond to these drugs or to evidence-based trauma-specific psychotherapy or cognitive-behavioral therapies. The FDA has designated MDMA-assisted therapy for PTSD a “Breakthrough Therapy.” This designation indicates that the therapy may be a substantial improvement over existing therapies for a serious condition, and is intended to speed up the drug development and review process.
MDMA (or 3,4-methylenedioxymethamphetamine) is the same chemical used recreationally under the name ecstasy or molly. However, the positive effects in the trial followed careful dosing (which is impossible with street drugs) and targeted therapy sessions.
Better One-Year Clinical Outcomes After Four Weeks of Theta Burst Stimulation for PTSD Than After Two Weeks
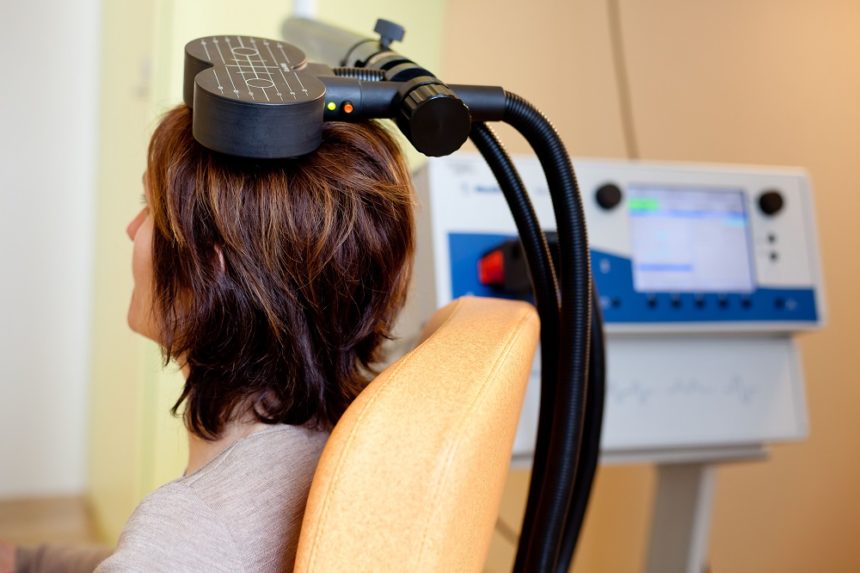
In a 2019 article in the journal Neuropsychopharmacology, Nicholas J. Petrosino and colleagues described findings from one year of follow-up with veterans suffering from post-traumatic stress disorder (PTSD) who received intermittent theta burst transcranial magnetic stimulation (iTBS) in a four-week crossover study.
In the first two weeks of the study, half of the 50 participants (who were mostly male and had an average age of 51) received iTBS while the others were given a sham procedure. Then all the participants received iTBS on an open (non-blind) basis for two more weeks.
At one month, those who had received four total weeks of iTBS had better outcomes than those who had received only two weeks of active iTBS. These results were published in the American Journal of Psychiatry in 2019 in an article by Noah S. Philip and colleagues.
The researchers went on to look at longer-term outcomes, namely time until relapse (a major event such as a re-hospitalization or suicide). After one year, those who received four weeks of iTBS went 9 to 11 months without relapsing (296.0 days ± 22.1), while those who received only two weeks of iTBS went 5 to 7 months before relapsing (182.0 days ± 31.9).
It seems that more iTBS may be better than less iTBS for PTSD in both the short and long term.
Prazosin Effective and Well-Tolerated for PTSD in Young People
In a poster session at the 2019 meeting of the American Academy of Child and Adolescent Psychiatry (AACAP), three posters highlighted the efficacy and tolerability of prazosin, a drug typically used to treat high blood pressure, for the treatment of childhood-onset post-traumatic stress disorder (PTSD).
Researchers Samira Khan and Taniya Pradhan of West Virginia University reviewed cases in which 39 patients aged 8–19 received 1–5 mg of prazosin at bedtime. The mean dose was 1.72 mg. Sleep (including nightmares) improved in 92.3% of the youths, and mood improved in 33.3%. Sleep improved more in patients who received lower doses (1–2 mg) than those who received higher doses. About 70% of the patients whose data were included in the case series were also receiving psychotherapy while being treated with prazosin.
In another poster, researcher Vladimir Ferrafiat and colleagues from University Hospital of Rouen in France reported on a prospective study of 18 participants under age 15 with severe PTSD who were unresponsive to other medications. The participants were given 1 mg of prazosin at bedtime, which was increased to 3 mg in 20% of the participants. The youth were assessed weekly over a one-month period. Improvement was seen in all domains, including sleep, nightmares and daytime intrusive symptoms. Prazosin was well tolerated, with only one patient experiencing low blood pressure, which did not necessitate withdrawal from the study.
In the final poster, researcher Fatima Motiwala and colleagues reviewed the literature on the treatment of PTSD in children. Motiwala indicated that among the options, prazosin was widely used in her hospital, at doses starting at 1 mg given at bedtime and increasing to a mean of 4 mg at bedtime with excellent results and tolerability.
Editor’s Note: Although these were not double-blind controlled studies, the findings are noteworthy in that they provide consistent data on the effectiveness and tolerability of prazosin in low doses in children with PTSD, essentially mirroring controlled data in adults, where higher doses are typically required.
Newly Identified Effects of N-Acetylcysteine
 In a talk at the 2019 meeting of the International Society for Bipolar Disorders, researcher Michael Berk, who was responsible for some of the initial findings on the effects of the antioxidant N-acetylcysteine (NAC), summarized some of the newer findings about the treatment.
In a talk at the 2019 meeting of the International Society for Bipolar Disorders, researcher Michael Berk, who was responsible for some of the initial findings on the effects of the antioxidant N-acetylcysteine (NAC), summarized some of the newer findings about the treatment.
NAC has been found to be effective in bipolar depression and in the treatment of both positive and negative symptoms of schizophrenia. It also helps in the avoidance of cocaine, alcohol, tobacco, and marijuana. It can reduce habitual behaviors such as gambling, obsessive compulsive disorder (OCD), and trichotillomania (compulsive hair-pulling) and irritability and motor stereotypy (repeated movements) in autism.
A 2016 study by researcher Sudie E. Back and colleagues in the Journal of Clinical Psychiatry found that NAC improved symptoms of post-traumatic stress disorder (PTSD) in veterans who also had depression and substance use disorders at a dosage of 2.4 grams/day.
According to Berk, NAC also reduces the incidence of lithium-related renal failure and reduces mitochrondrial toxicity. One study reported that it improved working memory in patients with schizophrenia.
In his talk, Berk also noted that statins offer an interesting new avenue for treatment. Several studies have suggested statins can improve mood or reduce the likelihood of a depressive recurrence. Angiotension-active drugs (inhibitors) have also been reported to decrease the incidence of depression and to improve cognition.
Recent Cannabis Use Linked to Greater Symptoms of Anxiety and Mood Disorders and Less Response to Treatment
 In a 2018 systematic literature review published in the Journal of Clinical Psychiatry, researcher George Mamman and colleagues reported that across 12 studies of people with anxiety and mood disorders, participants who had used cannabis in the previous six months had more symptoms than those who had used less cannabis or no cannabis during that period.
In a 2018 systematic literature review published in the Journal of Clinical Psychiatry, researcher George Mamman and colleagues reported that across 12 studies of people with anxiety and mood disorders, participants who had used cannabis in the previous six months had more symptoms than those who had used less cannabis or no cannabis during that period.
The 12 studies reviewed included a total of 11,959 participants. Four studies looked at post-traumatic stress disorder (PTSD), one at panic disorder, five at bipolar disorder, and 2 at depressive disorder. In addition to finding that recent cannabis use was associated with greater symptoms, the authors of the review also found that in 10 of the 12 studies, recent cannabis use was associated with less symptom improvement in response to treatment for bipolar disorder, depression, and PTSD; including both medication and psychotherapy.
In bipolar disorder, cannabis use was associated with greater symptom severity. Cannabis use for more than one year was linked to more recurrences of mania and shortened time to a recurrence. Compared to participants with no prior use of cannabis, those with a cannabis use disorder had more depressive symptoms, including sleep troubles and loss of interest in activities one had previously enjoyed.
In PTSD, any cannabis use at the beginning of the analysis period and sustained use of cannabis over time were both linked to greater symptom severity in the four months following the beginning of the analysis.
Mammen and colleagues cautioned that these results are limited based on the differences in measurements across the 12 studies, the inpatient populations under study, and the uncontrolled nature of the cannabis the participants accessed on their own time. However, the authors suggest that the findings may inform patients’ and doctors’ conversations about whether or not to use cannabis.
Repeated Ketamine Reduces PTSD and Depression in the Short Term
In a 2018 open study by C. Sophia Albott and colleagues in the Journal of Clinical Psychiatry, veterans with post-traumatic stress disorder (PTSD) and a simultaneous diagnosis of major depression were treated with six infusions of intravenous ketamine over a 12-day period (Mondays, Wednesdays, and Fridays for two weeks).
Ketamine produced large improvements in both conditions. The remission rate was 80.0% for PTSD and 93.3% for depression. The median time to first relapse after the treatment was 41 days for PTSD and 20 days for depression.
One side effect of ketamine was that dissociative symptoms increased temporarily with repeated infusions. PTSD symptoms did not worsen among those participants taking ketamine.
The study was intended to evaluate the efficacy, safety, and durability of repeated ketamine infusions. Ketamine has been used in emergency rooms to rapidly treat depression and suicidality, but the effects of a single infusion fade within days. Albott and colleagues reported that this treatment scenario with multiple ketamine infusions produced rapid results that lasted longer than single ketamine infusions.
Editor’s Note: While this study found that repeated ketamine infusions were safe, it is possible that long-term use may lead to addiction. Researcher Nolan R. Williams and colleagues reported in a 2018 article in the American Journal of Psychiatry that ketamine works via activation of the opiate receptor. The drug naloxone, which rapidly reverses opiate overdose, completely blocked ketamine’s antidepressant effects.
PTSD Increases Risk of Lupus
 A new large 2017 study in the journal Arthritis and Rheumatology reports that post-traumatic stress disorder (PTSD) in women triples their risk of developing the autoimmune disease lupus. The study included 54,763 civilian women whose health data was tracked over a period of 24 years.
A new large 2017 study in the journal Arthritis and Rheumatology reports that post-traumatic stress disorder (PTSD) in women triples their risk of developing the autoimmune disease lupus. The study included 54,763 civilian women whose health data was tracked over a period of 24 years.
Not only did PTSD increase lupus risk, but any traumatic event doubled lupus risk compared to women who were not exposed to trauma.
The researchers, led by Andrea L. Roberts, were taken by surprise at the strong links between trauma and lupus. Trauma was more of a risk factor for lupus than smoking.