Better One-Year Clinical Outcomes After Four Weeks of Theta Burst Stimulation for PTSD Than After Two Weeks
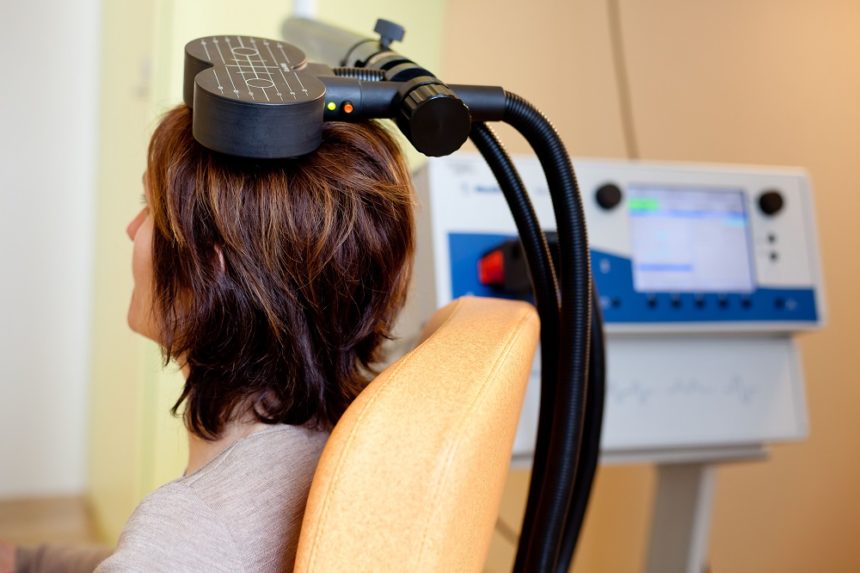
In a 2019 article in the journal Neuropsychopharmacology, Nicholas J. Petrosino and colleagues described findings from one year of follow-up with veterans suffering from post-traumatic stress disorder (PTSD) who received intermittent theta burst transcranial magnetic stimulation (iTBS) in a four-week crossover study.
In the first two weeks of the study, half of the 50 participants (who were mostly male and had an average age of 51) received iTBS while the others were given a sham procedure. Then all the participants received iTBS on an open (non-blind) basis for two more weeks.
At one month, those who had received four total weeks of iTBS had better outcomes than those who had received only two weeks of active iTBS. These results were published in the American Journal of Psychiatry in 2019 in an article by Noah S. Philip and colleagues.
The researchers went on to look at longer-term outcomes, namely time until relapse (a major event such as a re-hospitalization or suicide). After one year, those who received four weeks of iTBS went 9 to 11 months without relapsing (296.0 days ± 22.1), while those who received only two weeks of iTBS went 5 to 7 months before relapsing (182.0 days ± 31.9).
It seems that more iTBS may be better than less iTBS for PTSD in both the short and long term.
Medical Device May Treat Alzheimer’s Disease
A recently completed clinical trial suggests that NeuroAD, a treatment system that combines transcranial magnetic stimulation and cognitive training targeted at brain regions affected by Alzheimer’s disease, may be effective at treating mild to moderate cases of the illness.
Neuronix Ltd, the company that produces the device used to deliver transcranial magnetic stimulation in the trial, plans to seek Food and Drug Administration approval for NeuroAD. It would be the first device approved for the treatment of Alzheimer’s in the US. The device is already in use in Europe and Asia.
In the clinical trial, 131 patients received six weeks of the NeuroAD treatment or a sham treatment used as a comparison. Those participants who received the real intervention performed better on an assessment of Alzheimer’s and experienced minimal side effects.
In transcranial magnetic stimulation, a non-invasive procedure, magnets placed near the skull stimulate electrical impulses in the brain. This activates neurons, releasing excitatory transmitters and brain-derived neurotrophic factor (BDNF), which is important for new synapse formation and long-term learning and memory.
Editor’s Note: This editor (Robert Post) has long advocated the use of repeated transcranial magnetic stimulation (rTMS) with simultaneous cognitive behavioral or other positive therapy to activate and enhance specific neural circuits and relieve depression. The trial of NeuroAD adds evidence of the positive effects of this approach in domains other than depression. Cognitive training enhanced by rTMS may be helpful with a variety of cognitive difficulties.


