American Academy of Pediatrics Recommends Parents Avoid Spanking and Verbal Abuse
The American Academy of Pediatrics (AAP) has issued a policy statement calling for an end to corporal punishment, including spanking. These forms of punishment are tied to negative outcomes in every developmental area.
Children spanked regularly at age 3 had increased aggression risk by age 5. They also had more negative behaviors and lower vocabulary scores at age 9. Abusive behavior raises stress hormones and is associated with mental health struggles.
Verbal abuse should also be avoided. Verbal abuse includes punishment that shames, humiliates, threatens, frightens, or ridicules a child. Use of time outs, removal of privileges, and other forms of quiet discipline are recommended alternatives.
Editor’s Note: In our research network, the Bipolar Collaborative Network, we found that verbal abuse by itself (without the physical or sexual abuse that often accompany it) is associated with an earlier age of onset of bipolar disorder and a more difficult course of illness.
Family focused therapy (FFT) and other forms of family therapy are highly recommended for children of a parent with bipolar illness. These children are at high risk for a variety of psychiatric diagnoses, and those already experiencing depression, cyclothymia (mood swings between high and low) or a diagnosis of bipolar disorder not otherwise specified (BP-NOS) are much improved with FFT compared to treatment as usual. FFT teaches family members to recognize symptoms of illness for what they are rather than interpreting them as deliberate hostility, increases family communication and problem solving, and leads to good long-term outcomes.
Civil War Data Shows Father’s Trauma Can Affect Son’s Lifespan
An economist at the University of California Los Angeles (UCLA) has used Civil War data to determine that trauma experienced by a father can affect the lifespan of his son, but that a mother’s healthy diet during pregnancy can neutralize this risk.
Researcher Dora Costa used records from the National Archive to identify Union soldiers who were held as prisoners of war (POWs) by the Confederacy. She compared records of their children’s lifespans to the children of Union soldiers who were never held as POWs, finding that the sons of POWs were more likely to have died at any given age. (The study included only children who lived to be at least 45 years old.) Detailed records were kept because families of soldiers and POWs were eligible for generous pensions.
When looking at the data, Costa expected to find that socioeconomic status was the factor that explained discrepancies in lifespans among children of Civil War veterans. However, she noticed that the difference in lifespan only appeared in sons, and only to sons born after the war.
This pointed to an epigenetic explanation. Epigenetics is the idea that some aspects of a parent’s experiences (such as deprivation, drug use, etc.) can be passed on to their children during the gene transcription process. While a parent’s inherited genetic sequence doesn’t change, the structure of their DNA can be wound tightly or loosely depending on life experiences, and this affects how easily their genes are transcribed when passed on to their children.
The sons of POWs in the worst camp environments (typically during the later years of the war when prisoner exchanges were less frequent and overcrowding and malnutrition were common in camps) had even shorter lifespans than the sons of POWs who were imprisoned in less dire circumstances.
The research also looked at birth months to determine whether mothers would have had access to good nutrition while pregnant. Sons born to POW fathers in the later months of the year (whose mothers were likely to have had access to good nutrition) had lifespans comparable to the sons of non-POWs, while sons of POWs born earlier in the year fared worse.
The research was published in the journal Proceedings of National Academy of Sciences in 2018.
Editor’s Note: This is another example in humans of findings that have been clear-cut in animal studies. A father’s experiences, such as stressors or substance abuse, can influence the next generation even if the parent has no contact with the offspring. Epigenetic marks on DNA, histones (the structures around which DNA is wound), or microRNA of the sperm appear to carry these unexpected transgenerational effects.
Inflammation is Associated with Antidepressant Treatment Resistance
Researcher Ebrahim Haroon and colleagues report in a 2018 issue of Psychoneuroendocrinology that people whose depression failed to respond to at least three different antidepressants in their current episode of depression had higher levels of inflammation than those who had fewer than three failed antidepressant trials.
The researchers found that patients who had not responded to antidepressants had higher levels of the inflammatory markers TNF-alpha, TNF-alpha receptor 2, and Il-6. The inflammatory marker CRP was also significantly elevated in these patients when statistical analyses that excluded body mass index (BMI) were used.
Haroon and colleagues reported that a third of all patients with major depressive disorder fail to respond to currently available antidepressant treatments, and that inflammation may be to blame because it interferes with the neurotransmitter systems that antidepressants target.
Editor’s Note: These data indirectly support the use of anti-inflammatory drugs to augment the effects of antidepressants in patients with treatment resistant depression. A caution that may be very important is to assess evidence of inflammation at baseline, as some data suggest that people with low CRP may, for example, do more poorly with a blocker of TNF-alpha, while people with high CRP at baseline (over 3 pg/ml) show good improvement.
Inflammatory Marker IL-6 is Elevated in People with Depression and Those with a History of Childhood Trauma
In a 2018 article in the journal Psychiatry Research, researcher Ana Munjiza and colleagues reported that the inflammatory marker IL-6 was higher in 64 depressed people than in 53 non-depressed people, and that levels of IL-6 among people in the depressed group were significantly correlated with scores on a questionnaire in which participants reported traumas experienced in childhood. They reported more physical abuse, physical neglect, and emotional abuse.
Munjiza and colleagues indicate that trauma in childhood is a risk factor for depression in adulthood, as other researchers have suggested, and that inflammation could mediate the relationship between childhood adversity and depression.
Editor’s Note: IL-6 has been associated with antidepressant treatment resistance. IL-6 is secreted from white cells in the blood and from monocytes from the bone marrow in response to stress. It enters the brain and starts an inflammatory cascade that induces depressive behaviors. Animal studies have shown that if IL-6 secretion is blocked, depressive-like behaviors do not occur.
Another indicator of inflammation is CRP, and elevations in CRP have been associated with poor response to selective serotonin reuptake inhibitor (SSRI) antidepressants, and better response to the noradrenergic tricyclic antidepressant nortriptyline and the dopamine active antidepressant bupropion.
Treatments for depressed people with histories of childhood trauma may include psychotherapy, somatic therapies such as repeated transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), and medication. More research is needed to determine the optimal treatment regimens for this subgroup of depression sufferers, including whether anti-inflammatory drugs could play a helpful role in preventing or treating depression. People with elevated inflammatory markers (such as IL-6, CRP, IL-1, or TNF-alpha) are likely to be better candidates for adjunctive anti-inflammatory treatments than those with normal or low baseline levels of inflammation.
Inflammation and Depression: Treatment Implications
 Vladimir Maletic of the University of South Carolina School of Medicine Greenville gave a plenary talk at the 2018 meeting of the North Carolina Psychiatric Association that described a variety of ways that inflammation can drive depression.
Vladimir Maletic of the University of South Carolina School of Medicine Greenville gave a plenary talk at the 2018 meeting of the North Carolina Psychiatric Association that described a variety of ways that inflammation can drive depression.
Maletic explained that stress can increase neurotransmitters that activate brain macrophages, increase NFkB (a protein that controls DNA transcription and cell survival), and increase brain inflammation, evidenced by elevated levels of the inflammatory markers IL-1b, IL-6, TNF-alpha, and C-reactive protein (CRP). These signs of inflammation are associated with changes in brain function and connectivity that are consistent with depression, fatigue, and cognitive slowing.
Inflammation measured outside of the brain and spinal cord is associated with increased activity of the insula (a key brain sensor and modulator of emotions), disconnection between the prefrontal cortex and the reward circuits in the nucleus accumbens, and decreased function and structural changes to the hippocampus (critical for memory).
Maletic also explained that inflammation changes the way the amino acid tryptophan is metabolized. Normally tryptophan is converted into kyneurenic acid, which prevents excitotoxicity and has anticonvulsant effects. Stress can lead to tryptophan being metabolized instead into quinolinic acid, which is neurotoxic and has been linked to certain psychiatric disorders and neurodegenerative processes. This in turn impairs synaptic functioning, including increasing glutamate and decreasing brain-derived neurotrophic factor (BDNF), impairing a type of glia called oligodendroglia (which produce myelin), and the formation of new neural connections.
These findings have several important implications for treatment. Increases in inflammation have been linked to the atypical type of depression characterized by increased appetite, weight gain, and increased sleep rather than the more classic presentation of depression that includes loss of appetite, weight loss and insomnia. Thus, weight gain, waist circumference, and body mass index (BMI) are correlated with inflammation and can signal a poor response to medications (including the rapid-acting antidepressant ketamine and some other antidepressants). If someone with unipolar depression has high levels of CRP, they tend to have a poorer response to selective serotonin reuptake inhibitor (SSRI) antidepressants, and may respond better to the noradrenergic tricyclic antidepressant nortryptyline, the serotonin and norepinephrine reuptake inhibitors (SNRIs), and the dopamine active antidepressant bupropion.
There is some good news. Read more
Supplements for the Treatment of Schizophrenia
At the 2018 meeting of the North Carolina Psychiatric Association, researcher Karen Graham reviewed evidence for adjunctive treatments that may help treat schizophrenia when added to antipsychotic medications.
Graham endorsed omega-3-fatty acids, saying that they may delay the conversion to schizophrenia in young people at high risk for the illness. Data in chronic schizophrenia are more equivocal.
Data on the effects of vitamin D3 in schizophrenia are mixed, but D3 is often low in patients with psychotic disorders, and supplementation with vitamin D3 in the general population has been associated with decreases in cancer and all-cause mortality.
Graham indicated that in three studies vitamin B6 (pyridoxine) decreased tardive dyskinesia, a side effect of antipsychotic medication that is characterized by repetitive or jerky involuntary movements of the face and body. B6 also reduced the severity of akathisia or restless legs, which is comparable to the effects of 40mg/day of the beta blocker drug propranolol. Graham recommended a dose of 300mg/day of B6 that could be increased up to 600mg twice per day. The onset of effects usually begins by week three, and the cost ranges from 25 to 80 cents per day.
The antioxidant supplement N-acetylcysteine (NAC) may also help. Graham described six studies that found NAC had positive effects on negative symptoms (apathy, blunted emotions, etc.) and/or cognition in patients with schizophrenia. The dosage in these studies was usually 2 grams/day for 24 weeks. The cost was 50 cents per day.
Two 8-week trials of L-theanine (an amino acid found in green and black tea) at doses of 400mg/day improved negative symptoms and anxiety in 40 patients with schizophrenia. The rationale for the study was that L-theanine increases inhibitory neurotransmitters, modulates the amino acid 5-HTP and the neurotransmitter dopamine, increases brain-derived neurotrophic factor (BDNF), and may be neuroprotective after a heart attack or a traumatic brain injury. The cost is 40 cents per day.
Graham reported that the supplement ginkgo biloba produced significant improvement in negative symptoms and total symptoms in eight clinical trials that included a total of 1,033 patients with schizophrenia. Doses ranged from 240 to 360 mg/day. These supplements (usually extracted from leaves of the ginkgo tree) have not been found to have many side effects, but they can reportedly increase post-operative bleeding. Gingko biloba supplements cost 20 to 80 cents per day. There is also at least one positive study of ginkgo biloba in tardive dyskinesia.
Three of four studies of cannabidiol in schizophrenia have been positive (at doses of 600, 800, and 1,000 mg/day in studies that lasted four to six weeks). There are now six additional ongoing studies listed on the website clinicaltrials.gov. There is little of this diol component in regular marijuana, and the cost of pure cannabidiol is unfortunately an exorbitant $60 to $100/day.
There is a positive controlled study of the herb ashwagandha in 66 patients with schizophrenia.
Not included in Dr. Graham’s review was the prenatal treatment of women with phosphatidylcholine (900mg/day) followed by supplements in the newborn, which normalized an aspect of sensory gating known as P50 in patients with schizophrenia. Healthy individuals show a reduced response to an auditory cue when it is repeated 50 milliseconds after the initial cue. In people with schizophrenia, response to the repeated cue is not suppressed. This has been suggested by researchers Robert Freedman and Randal G. Ross in a 2015 article in the Shanghai Archives of Psychiatry as a possible primary preventive approach to schizophrenia.
Pregnant women in their second and third trimesters should at least consume foods high in choline, especially if the fetus is at high risk for schizophrenia because of a family history of schizophrenia.
Beef liver is very high in choline, providing 420mg per slice. Other animal products provide significant choline, such as eggs (120 mg/egg), beef (90mg/100g), chicken liver (85mg/liver), fish (85mg/100g), bacon (35mg/strip) or other pork, chicken (67mg/100g). Tofu (36mg/half cup) and cereal (22mg/half cup) are also sources of choline.
Foods High in Choline
| Beef liver | 1 slice | 420mg choline; |
| Egg | 1 egg | 120; |
| Beef | 100 gm | 90; |
| Chicken liver | 1 liver | 85; |
| Fish | 100 gm | 85; |
| Bacon or pork | 2 strips bacon | 70; |
| Chicken | 100 gm | 67; |
| Tofu | 120 ml (0.5 cup) | 36; |
| Cereal | 120 ml (0.5 cup) | 22 |
Using Light to Improve Sleep and Depression
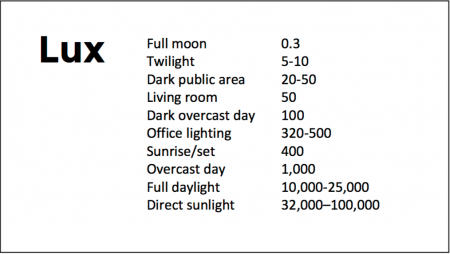
At the 2018 meeting of the North Carolina Psychiatric Association, researcher Chris Aiken described the phenomenon of sleep inertia, when people are awakened from deep sleep by an alarm, rather than waking at the end of a sleep cycle, and are groggy for 15 minutes. Depressed people may stay groggy for 4 hours. A dawn simulator may help. These lights turn on gradually over the course of 30 to 60 minutes, reaching 250 lux while the patient is still asleep. Dawn simulators have worked in eight out of ten controlled clinical trials to help people with seasonal affective disorder, adolescents, and normal adults wake up more easily. They range in cost from $25 to $90 and some brands include PER2LED or LightenUp. Aiken says dawn simulators can improve depression, sleep quality, and cognition.
Evening and nighttime light: Bright lights and blue light, like the light that comes from electronic screens, can shut down the body’s secretion of melatonin, making people awake and alert in the evening when they should be getting sleepy. Dim light or glasses that filter out blue light allow increases in melatonin secretion in the evening, while bright light suppresses it. Missing this early melatonin pulse creates “night owls” who have delayed sleep onset.
Because light still reaches our eyes through our eyelids as we sleep, even low-level light during the night impairs sleep, cognition, and learning, and increases the risk of depression by a hazard ratio of 1.8 (about double the risk). A 2017 study by Kenji Obayashi in the American Journal of Epidemiology found that bedroom light above 5 lux elevated rates of depression in older adults after two years of followup. Living room light averaged around 50 lux and increased depression further.
The treatment is turning off TVs, electronic screens, and cellphones in the evening or wearing blue-blocking glasses, which can be found for less than $10. Blue-blocking glasses can increase calmness and reduce anxiety, and even are effective in treating mania. Then, during sleep, wear an eye mask or get light-blocking blinds or curtains for windows. For a complete blackout, use blackout curtains, aluminum foil over windows, electric tape over LED lights, or try sleeping in the basement.
Aiken suggests that to re-instate healthy sleep patterns, people should institute virtual darkness from 6pm to 8am, including wearing blue-blocking glasses when out of bed. Then they should institute total darkness or wear an eye mask when in bed. When symptoms improve, this routine can gradually be shifted to begin later in the evening, such as two hours before bedtime.
Blue light filters are also available for smartphones and tablets including Apple Nightshift mode, Kindle BlueShade, and Android Twilight and Blue Light Filter.
Glasses that filter out blue light include Uvex Ultraspec 2000, 50360X ($7 on Amazon) and Uvex Skyper 351933X ($7-10 on Amazon). The website lowbluelights.com sells blue-blocking glasses from $45 and a variety of other blue-free lighting products such as lightbulbs and flashlights.
Bright light therapy for unipolar and bipolar depression: 30 minutes of bright light (7,500 to 10,000 lux) in the morning can help treat depression in unipolar and bipolar disorder and seasonal affective disorder. The effects usually take 3 to 7 days to set in, but they only last while a patient continues using the bright light in the morning. Researcher Dorothy K. Sit and colleagues found that bright light therapy in the morning sometimes caused hypomanic reactions in people with bipolar disorder, and reported in a 2018 article in the American Journal of Psychiatry that midday light therapy (from noon to 2:30pm) was also effective without this unwanted effect. However, a 2018 article by Ne?e Yorguner Küpeli and colleagues in the journal Psychiatry Research suggested that a half hour of morning light for two weeks was sufficient to bring about improvement in 81% of patients with bipolar disorder and did not cause serious side effects.
Melatonin regimen for sleep onset delay: Melatonin can be used to treat severe night-owls with a very late onset of sleep (for example, going to bed at 2 or 3am and sleeping late into the morning). Melatonin can help with sleep onset to some extent when used at bedtime, but in those with an extreme phase shift, researcher Alfred J. Lewy recommends a regimen of low dose priming with 400–500 micrograms of melatonin at 4pm and then a full dose of 3 milligrams of melatonin at midnight. The 4pm priming dose helps pull back the delayed onset of the body’s secretion of melatonin toward a more normal schedule.
Recent Cannabis Use Linked to Greater Symptoms of Anxiety and Mood Disorders and Less Response to Treatment
 In a 2018 systematic literature review published in the Journal of Clinical Psychiatry, researcher George Mamman and colleagues reported that across 12 studies of people with anxiety and mood disorders, participants who had used cannabis in the previous six months had more symptoms than those who had used less cannabis or no cannabis during that period.
In a 2018 systematic literature review published in the Journal of Clinical Psychiatry, researcher George Mamman and colleagues reported that across 12 studies of people with anxiety and mood disorders, participants who had used cannabis in the previous six months had more symptoms than those who had used less cannabis or no cannabis during that period.
The 12 studies reviewed included a total of 11,959 participants. Four studies looked at post-traumatic stress disorder (PTSD), one at panic disorder, five at bipolar disorder, and 2 at depressive disorder. In addition to finding that recent cannabis use was associated with greater symptoms, the authors of the review also found that in 10 of the 12 studies, recent cannabis use was associated with less symptom improvement in response to treatment for bipolar disorder, depression, and PTSD; including both medication and psychotherapy.
In bipolar disorder, cannabis use was associated with greater symptom severity. Cannabis use for more than one year was linked to more recurrences of mania and shortened time to a recurrence. Compared to participants with no prior use of cannabis, those with a cannabis use disorder had more depressive symptoms, including sleep troubles and loss of interest in activities one had previously enjoyed.
In PTSD, any cannabis use at the beginning of the analysis period and sustained use of cannabis over time were both linked to greater symptom severity in the four months following the beginning of the analysis.
Mammen and colleagues cautioned that these results are limited based on the differences in measurements across the 12 studies, the inpatient populations under study, and the uncontrolled nature of the cannabis the participants accessed on their own time. However, the authors suggest that the findings may inform patients’ and doctors’ conversations about whether or not to use cannabis.
Marijuana Use in Early Adolescence Triples Risk of Psychosis At Age 18
 Hannah J. Jones and colleagues reported in the journal JAMA Psychiatry in 2018 that early- and late-onset marijuana use increased the risk of psychosis at age 18 (odds ratio 3.7 to 2.97). Interestingly, early-onset cigarette use also increased risk of psychosis, but much of the link between cigarette use and psychosis disappeared after correcting for confounding variables.
Hannah J. Jones and colleagues reported in the journal JAMA Psychiatry in 2018 that early- and late-onset marijuana use increased the risk of psychosis at age 18 (odds ratio 3.7 to 2.97). Interestingly, early-onset cigarette use also increased risk of psychosis, but much of the link between cigarette use and psychosis disappeared after correcting for confounding variables.
The data on 5,300 participants born from 1991 to 1992 came from the Avon Longitudinal Study of Parents and Children. Researchers followed up with the participants about their use of marijuana and cigarettes at least three times between the ages of 14 and 19.
Editor’s Note: These data add to a host of epidemiological data that smoking marijuana doubles the risk of psychosis. Risk is further increased among people with a common genetic variant (val/val) of the gene for COMT (catechol-O-methyltransferase), which metabolizes prefrontal dopamine. The variant, which includes two valine amino acids, functions better than other variants that include methionine amino acids. People with val/met or met/met COMT genes metabolize dopamine more slowly, making them relatively protected.
The data are also pretty strong that early heavy use of marijuana is a risk factor for new onset of both bipolar disorder and schizophrenia (and not just an earlier onset in those who might have been vulnerable otherwise).
While marijuana use has become more mainstream with its legalization in many states, its recreational use still carries risks of mental illness. In addition to increasing psychosis risk, marijuana use can also make bipolar disorder more difficult to treat.
A minor component of marijuana, cannabidiol, can have some positive effects, but what you get most of when consuming marijuana is tetrahydrocannabinol (THC), which produces symptoms that resemble psychosis.
Data in rats indicate that a father rat’s use of THC as an adult increases the risk that his offspring (with which he has no contact) will be prone to opiate addiction. The effect is an epigenetic one, conveyed by chemical changes in the father’s DNA that get passed on to the next generation via changes that persist in his sperm. We don’t know if this also happens with humans. So even if you are not worried about your own health, avoiding marijuana use might be good for your children.
Pilot Study Finds Intravenous Ketamine Improves Tough-to-Treat Adolescent Depression
A 2018 open study by Kathryn R. Cullen and colleagues in the Journal of Child and Adolescent Psychopharmacology suggests that intravenous ketamine may improve depression in adolescents who have not responded to at least two antidepressants.
Thirteen patients ranging in age from 12 to 18 with treatment-resistant depression were given six ketamine infusions over a period of two weeks, at doses of 0.5 mg/kg of body weight. A 50% drop in scores on the Children’s Depression Rating Scale-Revised (CDRS-R) was considered a good response, and the average drop in participants’ scores was 42.5%. Five of the thirteen participants (38%) met the criteria for a good response. Three of these participants were still in remission at six weeks, while the other two relapsed within two weeks.
Ketamine was fairly well-tolerated by the young participants. Some had temporary dissociative symptoms or blood pressure changes. Higher absolute doses of ketamine were linked to better response.
The response rates in this group were not as good as in some studies of adults. More research using larger sample sizes and placebo controls is needed to optimize dosing and clarify the safety and efficacy of intravenous ketamine in adolescents with tough-to-treat depression, but this is a promising finding in a small number of adolescents.