Psilocybin Comparable to Escitalopram in the Treatment of Depression

Four randomized, controlled clinical trials have established that psilocybin, the hallucinogenic compound in “magic mushrooms,” has anti-depressant effects. Recently, a phase 2 clinical trial compared the effects of psilocybin to a US Food and Drug Administration (FDA)–approved treatment for depression, the selective serotonin-reuptake inhibitor (SSRI) escitalopram. The study by Robin Carhart-Harris and colleagues was published in the New England Journal of Medicine in 2021.
The 59 participants in the 6-week study, which took place in the United Kingdom, had longstanding moderate-to-severe major depressive disorder. They were randomized to two groups. The psilocybin group received two separate doses of 25 mg of psilocybin 3 weeks apart, plus 6 weeks of daily placebo. The escitalopram group received two separate doses of 1 mg of psilocybin 3 weeks apart plus 6 weeks of daily oral escitalopram. (The small doses of psilocybin administered to the escitalopram group were assumed to have negligible psychiatric effects.) All participants were told they would receive psilocybin (though not the dose) in order to standardize their expectations.
In addition to the drug treatments, all participants also received psychological support, which consisted of monitoring immediately after the administration of the drugs (given the expectation that the 25mg dose of psilocybin would produce an “altered quality of conscious experience”), psychological debriefings, and an active listening session.
Psilocybin improved depression symptoms to a greater extent than escitalopram did. Among the participants, 70% of the psilocybin group responded to the treatment, compared with 48% of the escitalopram group. Remission occurred in 57% of the psilocybin group compared with 28% of the escitalopram group. These differences in the outcomes for the two groups were not statistically significant.
The FDA has designated psilocybin a “Breakthrough Therapy” for severe depression, which indicates that the therapy may be a substantial improvement over existing therapies for a serious condition. The designation is intended to speed up the drug development and review process.
The state of Oregon legalized psilocybin-assisted therapy in 2020.
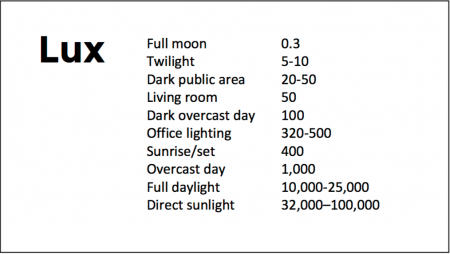
Good Outcomes Among Patients with Major Depressive Disorder Treated with Transcranial Magnetic Stimulation in a Large Study
At the 2021 meeting of the Society of Biological Psychiatry (SOBP), researcher Harold Sackeim and colleagues reported on data collected from patients in clinical treatment for major depression who received transcranial magnetic stimulation (TMS) at 103 practice sites. A total of 5,010 depressed patients were included in the intent-to-treat sample, and 3,814 completed the study, meaning that they either reached remission or were treated at least 20 times and went through a final assessment. “Response (58–83%) and remission (28–62%) rates were notably high across self-report and clinician-administered assessments,” and women had better outcomes than men. Sackeim and colleagues concluded, “Strong efficacy and the low side effect and medical risk profile suggest that TMS be evaluated as a first-line treatment for [major depressive disorder].”
ADHD Common in People with Mood Disorders

In a meta-analysis published in the journal Acta Psychiatrica Scandinavica in 2021, researcher Andrea Sandstrom and colleagues reported that people with mood disorders had a three times higher incidence of attention-deficit hyperactivity disorder (ADHD) than people without mood disorders. ADHD was also more likely to occur in people with bipolar disorder than in people with major depression. The comorbidity is most common in childhood, less so in adolescence, and lowest in adulthood.
Based on 92 studies including a total of 17,089 individuals, the prevalence of ADHD in people with bipolar disorder is 73% in childhood, 43% in adolescence, and 17% in adulthood. Data from 52 studies with 16,897 individuals indicated that prevalence of ADHD in major depressive disorder is 28% in childhood, 17% in adolescence, and 7% in adulthood.
Editor’s Note: A key implication of this research is that there is a huge overlap of bipolar disorder and ADHD in childhood, and that physicians need to specifically look for bipolar symptoms that are not common in ADHD to make a correct diagnosis. These include: brief or extended periods of mood elevation and decreased need for sleep in the youngest children; suicidal or homicidal thoughts and threats in slightly older children; hyper-sexual interests and actions; and hallucinations and delusions. When these are present, even when there are also clear-cut ADHD symptoms, a clinician must consider a diagnosis of bipolar disorder and treat the child with mood stabilizers prior to using stimulants or other traditional ADHD medications.
Conversely, physicians should be aware of the much lower incidence of ADHD in adolescents and adults with bipolar disorder. Here one should first make sure that the apparent ADHD symptoms of hyperactivity, inattention, poor concentration, etc. do not result from inadequately treated mania and depression, and if they do, treat these symptoms to remission prior to using traditional ADHD medications.
Pilot Study of Pramipexole for Anhedonia Symptoms in Unipolar Depression

At a recent scientific meeting, researcher Laura Hack described an open-label pilot study of pramipexole in participants with major depression including anhedonia, or inability to feel pleasure. Five participants with prominent anhedonia and major depression completed eight weeks of treatment with pramipexole, which activates dopamine D2 and D3 receptors. Four of the five who completed the study saw notable improvements in their anhedonia and quality of life.
The starting daily dose was 0.26 mg, which was increased every three days to a target dose of 2.0 mg per day. Two additional patients dropped out due to side effects (nausea, poor sleep, and headache).
Low activity in the ventral striatum, a part of the brain associated with decision-making and implicated in the brain’s reward system, correlated with the severity of the patients’ anhedonia. These results suggest that further studies are warranted.
Pramipexole has also shown positive effects in two small studies in bipolar depression. The drug is currently approved by the US Food and Drug Administration for the treatment of Parkinson’s disease and also works in restless legs syndrome.
Translocator Protein Levels in Brain Predict Response to Anti-Inflammatory Celecoxib in Major Depressive Disorder
Gliosis describes changes in glia that result from damage to the central nervous system. Researchers can use PET scans (positron emission tomography) to measure the extent of gliosis in the brain. But a new study explored whether these PET scans could instead be used to determine who might respond to a given medication.
Researcher Sophia Attwells and colleagues reported in the journal Biological Psychiatry in 2020 that people with high levels of translocator protein (TSPO), a measure of gliosis and inflammation, had a better antidepressant response to the anti-inflammatory drug celecoxib than patients who started out with lower levels of TSPO.
The study participants, who had treatment-resistant depression, all received 200mg of the anti-inflammatory drug celecoxib twice/day for eight weeks on an open (non-blind) basis. Before they began taking celecoxib, the participants received PET scans to measure translocator protein total distribution volume (TSPO VT) in the prefrontal cortex and the anterior cingulate cortex.
Patients with high levels of TSPO showed greater reductions in depression ratings over the course of the study than those with normal levels of TSPO at baseline.
Attwells and colleagues conclude that “this personalized medicine approach of matching a marker of gliosis to [an anti-inflammatory treatment] …should be applied in early development of novel therapeutics, in particular for [treatment-resistant depression].”
Editor’s Note: These findings are of considerable importance, as they are among the first to indicate that measures of inflammation may predict response to an anti-inflammatory medication such as celecoxib. In a 2013 article in the journal JAMA Psychiatry, Charles L. Raison and colleagues reported that patients with high levels of the peripheral inflammatory marker CRP saw marked improvement in their depression when they received the anti-inflammatory treatment infliximab while those with lower or normal levels of inflammation actually worsened.
Psychiatric Risks in Offspring of Parents with Bipolar/Unipolar Disorders
 At the 2019 meeting of the International Society for Bipolar Disorders, researcher Martin Preisig and colleagues from Lausanne, Switzerland reported on a longitudinal study of mood disorders in offspring of parents with bipolar disorder, unipolar depression, or no history of psychiatric illness. The study included 446 children (with an average age of 10.1 years at the beginning of the study), who participated for an average of 11.9 years.
At the 2019 meeting of the International Society for Bipolar Disorders, researcher Martin Preisig and colleagues from Lausanne, Switzerland reported on a longitudinal study of mood disorders in offspring of parents with bipolar disorder, unipolar depression, or no history of psychiatric illness. The study included 446 children (with an average age of 10.1 years at the beginning of the study), who participated for an average of 11.9 years.
Preisig and colleagues determined symptoms and other factors that preceded psychiatric illness. They found that bipolar disorder in the offspring was preceded by sub-threshold hypomania, major depression, and conduct disorder. Bipolar disorder in the offspring was also predicted by parental early-onset bipolar disorder.
Major depression was preceded by separation anxiety disorder, and witnessing violence or being a victim of sexual abuse.
Preisig and colleagues concluded that not only did bipolar disorder and major depressive disorder have different familial origins, they also had different antecedents and risk factors.
Using Light to Improve Sleep and Depression
At the 2018 meeting of the North Carolina Psychiatric Association, researcher Chris Aiken described the phenomenon of sleep inertia, when people are awakened from deep sleep by an alarm, rather than waking at the end of a sleep cycle, and are groggy for 15 minutes. Depressed people may stay groggy for 4 hours. A dawn simulator may help. These lights turn on gradually over the course of 30 to 60 minutes, reaching 250 lux while the patient is still asleep. Dawn simulators have worked in eight out of ten controlled clinical trials to help people with seasonal affective disorder, adolescents, and normal adults wake up more easily. They range in cost from $25 to $90 and some brands include PER2LED or LightenUp. Aiken says dawn simulators can improve depression, sleep quality, and cognition.
Evening and nighttime light: Bright lights and blue light, like the light that comes from electronic screens, can shut down the body’s secretion of melatonin, making people awake and alert in the evening when they should be getting sleepy. Dim light or glasses that filter out blue light allow increases in melatonin secretion in the evening, while bright light suppresses it. Missing this early melatonin pulse creates “night owls” who have delayed sleep onset.
Because light still reaches our eyes through our eyelids as we sleep, even low-level light during the night impairs sleep, cognition, and learning, and increases the risk of depression by a hazard ratio of 1.8 (about double the risk). A 2017 study by Kenji Obayashi in the American Journal of Epidemiology found that bedroom light above 5 lux elevated rates of depression in older adults after two years of followup. Living room light averaged around 50 lux and increased depression further.
The treatment is turning off TVs, electronic screens, and cellphones in the evening or wearing blue-blocking glasses, which can be found for less than $10. Blue-blocking glasses can increase calmness and reduce anxiety, and even are effective in treating mania. Then, during sleep, wear an eye mask or get light-blocking blinds or curtains for windows. For a complete blackout, use blackout curtains, aluminum foil over windows, electric tape over LED lights, or try sleeping in the basement.
Aiken suggests that to re-instate healthy sleep patterns, people should institute virtual darkness from 6pm to 8am, including wearing blue-blocking glasses when out of bed. Then they should institute total darkness or wear an eye mask when in bed. When symptoms improve, this routine can gradually be shifted to begin later in the evening, such as two hours before bedtime.
Blue light filters are also available for smartphones and tablets including Apple Nightshift mode, Kindle BlueShade, and Android Twilight and Blue Light Filter.
Glasses that filter out blue light include Uvex Ultraspec 2000, 50360X ($7 on Amazon) and Uvex Skyper 351933X ($7-10 on Amazon). The website lowbluelights.com sells blue-blocking glasses from $45 and a variety of other blue-free lighting products such as lightbulbs and flashlights.
Bright light therapy for unipolar and bipolar depression: 30 minutes of bright light (7,500 to 10,000 lux) in the morning can help treat depression in unipolar and bipolar disorder and seasonal affective disorder. The effects usually take 3 to 7 days to set in, but they only last while a patient continues using the bright light in the morning. Researcher Dorothy K. Sit and colleagues found that bright light therapy in the morning sometimes caused hypomanic reactions in people with bipolar disorder, and reported in a 2018 article in the American Journal of Psychiatry that midday light therapy (from noon to 2:30pm) was also effective without this unwanted effect. However, a 2018 article by Ne?e Yorguner Küpeli and colleagues in the journal Psychiatry Research suggested that a half hour of morning light for two weeks was sufficient to bring about improvement in 81% of patients with bipolar disorder and did not cause serious side effects.
Melatonin regimen for sleep onset delay: Melatonin can be used to treat severe night-owls with a very late onset of sleep (for example, going to bed at 2 or 3am and sleeping late into the morning). Melatonin can help with sleep onset to some extent when used at bedtime, but in those with an extreme phase shift, researcher Alfred J. Lewy recommends a regimen of low dose priming with 400–500 micrograms of melatonin at 4pm and then a full dose of 3 milligrams of melatonin at midnight. The 4pm priming dose helps pull back the delayed onset of the body’s secretion of melatonin toward a more normal schedule.
Pilot Study Finds Intravenous Ketamine Improves Tough-to-Treat Adolescent Depression
A 2018 open study by Kathryn R. Cullen and colleagues in the Journal of Child and Adolescent Psychopharmacology suggests that intravenous ketamine may improve depression in adolescents who have not responded to at least two antidepressants.
Thirteen patients ranging in age from 12 to 18 with treatment-resistant depression were given six ketamine infusions over a period of two weeks, at doses of 0.5 mg/kg of body weight. A 50% drop in scores on the Children’s Depression Rating Scale-Revised (CDRS-R) was considered a good response, and the average drop in participants’ scores was 42.5%. Five of the thirteen participants (38%) met the criteria for a good response. Three of these participants were still in remission at six weeks, while the other two relapsed within two weeks.
Ketamine was fairly well-tolerated by the young participants. Some had temporary dissociative symptoms or blood pressure changes. Higher absolute doses of ketamine were linked to better response.
The response rates in this group were not as good as in some studies of adults. More research using larger sample sizes and placebo controls is needed to optimize dosing and clarify the safety and efficacy of intravenous ketamine in adolescents with tough-to-treat depression, but this is a promising finding in a small number of adolescents.
Meta-Analysis Finds Antidepressants More Effective Than Placebo
In a 2018 article in the journal The Lancet, researchers led by Andrea Cipriani compared the efficacy of 21 different antidepressants and established that antidepressants are more effective than placebo at reducing unipolar depression. To date, this is the largest meta-analysis of double-blind, randomized controlled studies of antidepressant efficacy, including 522 trials and a total of 116,477 participants. All 21 of the antidepressants were found to be more effective than placebo.
Looking at head to head studies, Cipriani and colleagues found that the most effective antidepressants were agomelatine, amitriptyline, escitalopram, mirtazapine, paroxetine, venlafaxine, and vortioxetine. The least effective antidepressants were fluoxetine, fluvoxamine, reboxetine, and trazodone.
In terms of tolerability, agomelatine, citalopram, escitalopram, fluoxetine, sertraline, and vortioxetine were most tolerable to patients, while amitriptyline, clomipramine, duloxetine, fluvoxamine, reboxetine, trazodone, and venlafaxine caused the most study dropouts due to side effects. Only agomelatine and fluoxetine had better dropout rates than placebo.
Interestingly, agomelatine, the medication found to be most effective and most tolerable, is unavailable in the US. Pharmaceutical company Novartis, which owns the rights to the drug, was disappointed by some lackluster studies of the drug and never applied for Food and Drug Administration approval to sell it in the US. The studies found potential problems regarding drug interactions related to the metabolic enzyme CYP1A2 and a risk of liver damage with longer-term use.
Editor’s Note: This meta-analysis should end any remaining controversy about the efficacy of antidepressants in the acute treatment of unipolar depression.
This study did not address maintenance treatment for the prevention of depressive episodes. Researcher John R. Geddes and colleagues have found robust, statistically significant data that continuation treatment with antidepressants can prevent depressive relapse, suggesting that if patients continue taking effective antidepressants, rather than switching to placebo, the antidepressants can reduce depressive occurrences by about 70%.
It is now recommended in most guidelines that patients with two or three prior episodes of depression consider staying on antidepressants indefinitely over their lifetime in order to prevent recurrence. Antidepressants increase the creation of new neurons and brain-derived neurotrophic factor (BDNF), which protects neurons and is important for learning and memory. Antidepressants can also prevent loss of hippocampal volume.
Vortioxetine Improves Processing Speed in Depression
 In May 2018, the US Food and Drug Administration (FDA) approved a label change for the antidepressant vortioxetine (Trintellix), reflecting new data that show the drug can improve processing speed, an aspect of cognitive function that is often impaired in people with depression. Vortioxetine was first approved by the FDA for the treatment of depression in 2013.
In May 2018, the US Food and Drug Administration (FDA) approved a label change for the antidepressant vortioxetine (Trintellix), reflecting new data that show the drug can improve processing speed, an aspect of cognitive function that is often impaired in people with depression. Vortioxetine was first approved by the FDA for the treatment of depression in 2013.
The approval followed eight-week double-blind placebo-controlled studies of vortioxetine’s effects on cognitive function in adults aged 18–65 who have depression. The studies were known as FOCUS and CONNECT. Patients received either 10mg/day, 20mg/day, or placebo. Those who took vortioxetine showed improvement on the Digit Symbol Substitution Test, a measure of processing speed, in addition to improvement in their depression.
Editor’s Note: This is the first time the FDA has approved labeling that describes an antidepressant as improving aspects of cognition in depression. Cognition is impaired in many patients with depression, such that this component of the drug’s effects could be of clinical importance. Among the 5 serotonin (5HT) receptor effects of the drug (in addition to the traditional blockade of serotonin reuptake shared by all selective serotonin reuptake inhibitor antidepressants (SSRIs)), it is likely that vortioxetine’s effects in blocking 5HT-3 and 5HT-7 receptors are important to the drug’s effects on processing speed.