Nutritional Supplement ALC Improves Depression
A meta-analysis of 12 studies suggests that the nutrient acetyl-l-carnitine (ALC), when taken as a nutritional supplement, has antidepressant effects. The meta-analysis by researcher Nicola Veronese and colleagues appeared in the journal Psychosomatic Medicine in 2017. Veronese and colleagues found that in nine randomized controlled trials, ALC reduced depressive symptoms significantly compared to placebo. In three randomized controlled trials that compared ALC with established antidepressants, ALC showed similar effectiveness at reducing depressive symptoms while producing 79% fewer side effects. Doses of ALC ranged from 1 to 4 grams per day, and higher doses led to greater improvement.
In the comparisons with antidepressants, the other treatments included fluoxetine (Prozac), duloxetine (Cymbalta), and amisulpride (which is not approved by the US Food and Drug Administration).
Low ALC has been linked to depression. According to Veronese and colleagues, ALC deficiency can dysregulate the transport of fatty acids across the inner membrane of mitochondria. The researchers suggest several ways that ALC might contribute to an improvement in depression. One is that is seems to promote neuroplasticity in cerebral regions implicated in depression, such as the hippocampus. It could also work by increasing brain-derived neurotrophic factor (BDNF), which protects neurons and is important for learning and memory. ALC decreases release of the neurotransmitter glutamate by increasing the production of the inhibitory metabotrophic glutamate receptor (mGluR-2) on presynaptic glutamate neurons . Another way ALC might work is by normalizing lipid metabolism. Or it could modulate neurotransmitters, increasing serotonin and dopamine and protecting against stress.
In the meta-analysis, ALC produced more improvement in older patients than in younger ones. The researchers stressed the need for better treatments for older people, which may experience falls, cardiovascular disease, or increased mortality from antidepressants.
ALC also seems to improve pain syndromes, making it a good option for patients with both depression and pain symptoms.
Veronese and colleagues cited another meta-analysis that found that taking ALC in addition to an antidepressant led to lower rates of adverse events than the antidepressants alone, which helped patients adhere to their drug regimen.
In Animals, Exposure to High Fat Diet During Pregnancy Can Affect Offspring’s Neurological Development
New research in non-human primates suggests that exposure to a high fat diet during pregnancy and in early development prior to weaning can increase the offspring’s propensity for anxiety later in life.
The new research echoes 2010 findings about rats. Researcher Staci D. Bilbo and colleagues reported in the journal of the Federation of American Societies for Experimental Biology that in rats, a high fat diet during pregnancy and lactation led to offspring with greater body weight, increased inflammation, and problems with anxiety and spatial learning. Switching to a standard diet after weaning did not eliminate these outcomes.
The recent research by Jacqueline R. Thompson and colleagues, published in the journal Frontiers in Endocrinology in July 2017, suggests that maternal nutrition in the primate during pregnancy and lactation can have long-lasting effects on offspring’s neurological development, altering the brain and endocrine system. These changes occurred even if the offspring began a normal diet after weaning.
65 female Japanese macaques were divided into two groups, one that received a high-fat diet and one that received a normal diet. In the offspring of mothers who ate a high-fat diet, the researchers found impaired development of neurons containing serotonin. The offspring of the high-fat diet group also showed behavioral alterations such as increased anxiety.
The high rates of obesity in the US and other developed nations make these findings particularly important. The researchers suggest that 64% of women in the US who are of reproductive age are overweight, and 35% are obese. Co-author Elinor Sullivan suggested that the findings from the study could motivate mothers to make healthy nutritional decisions, not only for themselves but for their children as well.
Phthalates in Plastics and Creams Cause Epigenetic Changes to Sperm
A recent study suggests that chemicals called phthalates that are used to make plastic flexible and to improve the texture of lotions, creams, and powders have effects on human sperm. Phthalates have become common in our environment since the invention of plastics, and most people have detectable levels of phthalate metabolites in their bodies.
The study, published by Haotian Wu and colleagues in the journal Human Reproduction in 2017, measured DNA methylation in a group of men’s sperm and compared this to levels of phthalate metabolites in the men’s urine.
DNA methylation changes the structure of a DNA strand. Extra methyl groups are attached to the strand, affecting the way it is transcribed, even though the inherited genetic sequence on the DNA strand remains the same. Changes like these to the structure of DNA and histones, which give DNA its helix shape, are known as epigenetic changes.
Wu and colleagues found 131 regions of DNA methylation in the men’s sperm that they could link to at least one of the phthalate metabolites found in the men’s urine.
Sperm takes 72 days to mature. Wu and colleagues suggest that exposure to phthalates in plastics or personal care products during this period may cause alterations to sperm, which could potentially affect the ease of conception or the development of potential offspring. The changes the researchers observed affected genes related to growth, development, and cellular function and maintenance.
In addition to chemical exposure, stressors and drug use can also bring about epigenetic changes to sperm. A father’s offspring may then have altered risk of drug use or other behaviors as a result of these epigenetic changes.
Phthalates, which can disrupt the endocrine system, have previously been found to alter men’s hormone levels and to hurt sperm quality. This is the first study to find that in people, phthalate concentrations measured before conception are associated with DNA methylation in sperm. This was a fairly small study in 48 men, and it remains to be studied whether the changes to sperm affect the offspring’s prenatal and early childhood development.
In addition to their presence in flexible plastics, phthalates may also be found in products such as shaving cream, shampoo, soaps, and detergents.
Using Antidepressants During Pregnancy Likely Does Not Increase Autism Risk
In the past year or so, several meta-analyses have analyzed data from numerous studies of a possible link between antidepressant use in pregnancy and autism in the offspring. In a 2017 article in the Journal of Clinical Psychiatry, researcher Chittaranjan Andrade offers a meta-analysis of these previous meta-analyses, and determines that while there is a small link between antidepressant use in pregnancy and autism in the offspring, it is most likely the mother’s depressive illness rather than the medications that is responsible for this link.
Andrade found that antidepressant exposure was linked to an increased risk of autism spectrum disorders in the offspring even when the antidepressant use occurred only before conception occurred, when it could not possibly have affected the future fetus’ physiology. This implies that it is the mother’s illness rather than the antidepressant treatment that is a determinant of autism risk.
Atypical Antipsychotic Drug Aripiprazole Appropriate for Pregnancies
 A 2017 systematic review in the Journal of Affective Disorders found that the atypical antipsychotic medication apripiprazole (Abilify) was relatively safe for use during pregnancy and lactation. Researcher Alessandro Cuomo and colleagues reviewed 93 articles from the last two decades of research.
A 2017 systematic review in the Journal of Affective Disorders found that the atypical antipsychotic medication apripiprazole (Abilify) was relatively safe for use during pregnancy and lactation. Researcher Alessandro Cuomo and colleagues reviewed 93 articles from the last two decades of research.
Placebo-controlled research on medications used during pregnancy are uncommon, due to ethical reservations about assigning women randomly to each group when their fetus may be affected. However, Cuomo and colleagues were able to find some large prospective studies and large database studies that shed light on aripiprazole’s safety during pregnancy. They concluded that the data on aripiprazole during pregnancy and breastfeeding were “relatively reassuring” and that the benefits of aripiprazole outweigh the potential risks.
Risks of relapse upon discontinuing a mood stabilizer can be as high as 80%. Illness in the mother conveys risks to the fetus, so the risk-benefit ratio may suggest that staying on effective aripiprazole treatment during pregnancy and lactation makes sense for many patients.
In a comment on the study reported by Reuters Health, Dr. Jennifer L. Payne of the Johns Hopkins School of Medicine said, “The main reason to discontinue aripiprazole for pregnancy…would be if it is not working and the mother is actively ill, or if she insisted on doing so. In my mind, the literature supports the use of aripiprazole during pregnancy in mothers with serious mental illness who are responding well to the medication.”
Folate Supplements Reduce Autism Rates in Offspring of Women Taking Anti-Epileptic Drugs During Pregnancy
 A 2017 study form Norway suggests that the offspring of women taking anti-epileptic drugs during pregnancy are less likely to develop autism if the women also take folic acid supplements.
A 2017 study form Norway suggests that the offspring of women taking anti-epileptic drugs during pregnancy are less likely to develop autism if the women also take folic acid supplements.
The study by Marte Bjørk and colleagues in the journal JAMA Neurology used data from 104,936 children aged 18 to 36 months. Those whose mothers took anti-epileptic drugs during pregnancy had elevated autism rates, but only if their mothers did not use folic acid supplements. The mothers’ folate levels in weeks 17 to 19 of their pregnancies were inversely related to the degree of autistic traits in their offspring.
Women without epilepsy and women whose epilepsy went untreated during pregnancy had children with similarly low rates of autism to those whose mothers supplemented their anti-epileptic medications with folic acid during pregnancy.
Several Studies Find Lamotrigine is Safe in Pregnancy
 Several studies have now suggested that lamotrigine is safe to use during pregnancy. In June 2017, researcher Gali Pariente and colleagues published a systematic review and meta-analysis in the journal CNS Drugs in which they reported that across 21 studies, lamotrigine use during pregnancy was not linked to an increase in birth defects.
Several studies have now suggested that lamotrigine is safe to use during pregnancy. In June 2017, researcher Gali Pariente and colleagues published a systematic review and meta-analysis in the journal CNS Drugs in which they reported that across 21 studies, lamotrigine use during pregnancy was not linked to an increase in birth defects.
In November 2017, a small study from an Israeli medical center reported data from 83 women who received lamotrigine during their first trimester of pregnancy. The study by Merav Cohen-Israel and colleagues in the British Journal of Clinical Pharmacology found that lamotrigine use was not linked to congenital malformations, neurodevelopmental disorders, or withdrawal symptoms in the offspring.
Of the 83 women, 76 received lamotrigine alone, four received it in combination with clonazepam, two with carbamazepine, and one in combination with both levetiracetam and phenytoin.
Management of Unipolar and Bipolar Depression During Pregnancy
 At the Maryland Psychiatric Research Society’s continuing medical education conference in November, Lauren Osbourne, Assistant Director of the Women’s Mood Disorders Clinic at Johns Hopkins Hospital, gave a presentation on the management of mood and anxiety during pregnancy and lactation. She had a number of important ideas for physicians and patients to consider in their decision-making process.
At the Maryland Psychiatric Research Society’s continuing medical education conference in November, Lauren Osbourne, Assistant Director of the Women’s Mood Disorders Clinic at Johns Hopkins Hospital, gave a presentation on the management of mood and anxiety during pregnancy and lactation. She had a number of important ideas for physicians and patients to consider in their decision-making process.
According to Osbourne, 60%-70% of pregnant women with unipolar depression who discontinue their antidepressants relapse. Of those with bipolar disorder who discontinue their mood stabilizers, 85% relapse, while 37% of those who stay on their medications relapse.
Something to consider when deciding whether to continue medication while pregnant is that depression in pregnancy carries its own risks for the fetus. These include preterm delivery, low birth weight, poor muscle tone, hypoactivity, increased cortisol, poor reflexes, and increased incidence of attention deficit hyperactivity disorder (ADHD) and other behavioral disorders.
The placenta makes an enzyme 11-BHSD2 that lowers the stress hormone cortisol in the baby. However, this enzyme is less active in depression, exposing the fetus to higher levels of cortisol.
Thus, the decision about whether to continue medications during pregnancy should consider the risks to the fetus of both the mother’s depression and the mother’s medications.
Most antidepressants are now considered safe during pregnancy. There have been reports of potential problems, but these data are often confounded by the fact that women with more severe depression are more likely to require antidepressants, along with other risk variables such as smoking or late delivery (after 42 weeks). When these are accounted for by using matched controls, the apparent risks of certain antidepressants are no longer significant. This includes no increased risk of persistent pulmonary hypertension, autism, or cardiac malformations.
There may be a possible increased risk of Neonatal Adaption Syndrome (NAS) in the first weeks of life in babies who were exposed to selective serotonin reuptake inhibitor (SSRI) antidepressants in the third trimester. This syndrome presumably results from antidepressant withdrawal, and can include respiratory distress, temperature changes, decreased feeding, jitteriness/irritability, floppiness or rigidity, hypoglycemia, and jaundice. There is not yet a robust literature on the syndrome, but Osbourne suggested that it disappears within 2 weeks of birth.
In her practice, Osbourne prefers to prescribe sertraline, which has the best safety data, along with fluoxetine. Sertraline is also OK for breastfeeding. There is less data on bupropion, but it also appears to be safe during pregnancy. Endocrine and enzyme changes in pregnancy typically cause a 40% to 50% decrease in concentrations of antidepressants, so doses of antidepressants typically must be increased in order to maintain their effectiveness.
Osbourne ranked mood stabilizers for bipolar disorder, from safest to most worrisome. Lamotrigine is safest. There is no evidence linking it to birth defects, but higher doses are required because of increased clearance during pregnancy. Lithium is next safest. There are cardiac risks for one in 1,200 patients, but these can be monitored. Carbamazepine is third safest. One percent of babies exposed to carbamazepine will develop spina bifida or craniofacial abnormalities. Valproate is least safe during pregnancy. Seven to ten percent of babies exposed to valproate will develop neural tube defects, other malformations, or developmental delay, with a mean decrease of 9 IQ points. The atypical antipsychotics all appear safe so far.
Alternatives and Adjuncts to Medications in Pregnancy
30 Minutes of TDCS Better Than 20 Minutes in Patients with Unipolar Depression
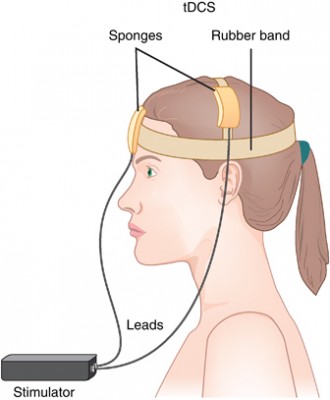 Transcranial direct current stimulation (tDCS) has successfully been used to treat depression. In this treatment, electrodes applied to the scalp provide a constant low level of electricity that can modulate neuron activity. In a 2017 article in the journal Progress in Neuro-Psychopharmacology & Biological Psychiatry, researcher Elena L. Pavlova and colleagues report that both 20- and 30-minute sessions of tDCS improved mild to moderate depression when combined with the selective-serotonin reuptake inhibitor (SSRI) antidepressant sertraline. However, the 30-minute sessions produced more improvement in depression.
Transcranial direct current stimulation (tDCS) has successfully been used to treat depression. In this treatment, electrodes applied to the scalp provide a constant low level of electricity that can modulate neuron activity. In a 2017 article in the journal Progress in Neuro-Psychopharmacology & Biological Psychiatry, researcher Elena L. Pavlova and colleagues report that both 20- and 30-minute sessions of tDCS improved mild to moderate depression when combined with the selective-serotonin reuptake inhibitor (SSRI) antidepressant sertraline. However, the 30-minute sessions produced more improvement in depression.
In the study, 69 right-handed patients (average age 37.6) received 50 mg of sertraline (Zoloft) per day and were randomized to one of three tDCS conditions: 10 daily 30-minute sessions, 10 daily 20-minute sessions, or 10 daily sham sessions with no tDCS treatment. The tDCS consisted of 0.5mA anodal current to the left dorsolateral prefrontal cortex.
Both 30-minute and 20-minute tDCS sessions produced greater benefit than the sham sessions. The 30-minute group showed significantly greater percentage improvement in depression scores than the 20-minute group, and included more participants who responded to treatment (89% compared to 68% of the 20-minute group and 50% of the sham group) and more whose depression remitted (70% compared to 27% of the 20-minute group and 35% of the sham group).