RTMS Study Identifies Glutamate as a Biomarker for Depression Treatment
 At the 3rd Annual Meeting of the Transcranial Magnetic Stimulation Society, Canadian researcher Frank MacMaster discussed his study of repeated transcranial magnetic stimulation (rTMS) in 50 children with depression. RTMS is a non-invasive procedure in which an electromagnetic coil is placed against the side of the forehead and magnetic pulses that can penetrate the scalp are converted into small electrical currents that stimulate neurons in the brain. The study was designed to identify biomarkers, or characteristics that might indicate which patients were likely to respond to the treatment. All of the patients received rTMS at a frequency of 10 Hz. Using magnetic resonance spectroscopy (MRS) technology, MacMaster found that children who responded well to rTMS treatment had low levels of the neurotransmitter glutamate at the beginning of the study, but their glutamate levels increased as their depression improved. Children who didn’t improve had higher glutamate levels at the beginning of the study, and these fell during the rTMS treatment.
At the 3rd Annual Meeting of the Transcranial Magnetic Stimulation Society, Canadian researcher Frank MacMaster discussed his study of repeated transcranial magnetic stimulation (rTMS) in 50 children with depression. RTMS is a non-invasive procedure in which an electromagnetic coil is placed against the side of the forehead and magnetic pulses that can penetrate the scalp are converted into small electrical currents that stimulate neurons in the brain. The study was designed to identify biomarkers, or characteristics that might indicate which patients were likely to respond to the treatment. All of the patients received rTMS at a frequency of 10 Hz. Using magnetic resonance spectroscopy (MRS) technology, MacMaster found that children who responded well to rTMS treatment had low levels of the neurotransmitter glutamate at the beginning of the study, but their glutamate levels increased as their depression improved. Children who didn’t improve had higher glutamate levels at the beginning of the study, and these fell during the rTMS treatment.
MacMaster hopes that glutamate levels and other biological indicators such as inflammation will eventually pinpoint which treatments are likely to work best for children with depression. At the meeting, MacMaster said that in Canada, only a quarter of the 1,200,000 children with depression receive appropriate treatment for it. Very little funding is devoted to research on children’s mental health, a serious deficit when one considers that most depression, anxiety, attention deficit hyperactivity disorder (ADHD), bipolar disorder, oppositional behavior, conduct disorder, and substance abuse begins in childhood and adolescence, and early onset of these illnesses has been repeatedly linked to poorer outcomes.
Editor’s Note: The strategy of identifying biomarkers is an important one. MacMaster noted that this type of research is possible due to the phenomenal improvements in brain imaging techniques that have occurred over the past several decades. These techniques include magnetic resonance imaging (MRI) to a resolution of 1 mm; functional MRI; diffusion tensor imaging (DTI), which can depict the connectivity of white matter tracts; and spectroscopy, which can be used to identify chemical markers of neuronal health and inhibitory and excitatory neurotransmitters, and analyze membrane integrity and metabolic changes. These methods provide exquisite views of the living brain, the most complicated structure in the universe. The biomarkers these techniques may identify will allow clinicians to predict how a patient will respond to a given treatment, to choose treatments more rapidly, and to treat patients more effectively.
Most SSRIs Free of Birth Defect Risk Early in Pregnancy, Fluoxetine and Paroxetine are Exceptions
A large study of women who took selective serotonin reuptake inhibitor (SSRI) antidepressants in the month before pregnancy and throughout the first trimester suggests that there is a smaller risk of birth defects associated with SSRI use than previously thought, though some risks were elevated in women who took paroxetine or fluoxetine.
The 2015 study, by Jennita Reefhuis and colleagues in the journal BMJ, investigated the drugs citalopram, escitalopram, fluoxetine, paroxetine, and sertraline, and examined birth defects that had previously been associated with SSRI use in smaller studies. The participants were 17,952 mothers of infants with birth defects and 9,857 mothers of infants without birth defects who had delivered between 1997 and 2009.
Sertraline was the most commonly used SSRI among the women in the study. None of the birth defects included in the study were associated with sertraline use early in pregnancy. The study found that some birth defects were 2 to 3.5 times more likely to occur in women who had taken fluoxetine or paroxetine early in their pregnancies.
Five different birth defects, while uncommon, were statistically linked to paroxetine use: anencephaly (undersized brain), heart problems including atrial septal defects and right ventricular outflow tract obstruction defects, and defects in the abdominal wall including gastroschisis and omphalocele. Two types of birth defects were associated with fluoxetine use: right ventricular outflow tract obstruction defects and craniosynostosis (premature fusion of the skull bones). Absolute incidence of these defects was also low.
RTMS and Other Treatments for Depression in Pregnancy
At the May meeting of the Society of Biological Psychiatry, researcher Deborah Kim gave a talk on the use of repeated transcranial magnetic stimulation (rTMS) for depression in women who are pregnant. In rTMS treatment, an electromagnetic coil is placed against the side of the forehead and magnetic pulses that can penetrate the scalp are converted into small electrical currents that stimulate neurons in the brain. Kim had recently completed an open study of rTMS in pregnant women, in which 70% of the women responded to rTMS. In another controlled randomized study of 30 women (also by Kim), 75% responded to active rTMS and 50% responded to a sham procedure. None of the women included had problems with the fetus or during delivery.
RTMS offers an alternative to women who are reluctant to take antidepressants during pregnancy. Kim cited data by Lee S. Cohen and colleagues in which women taking antidepressants show a 68% relapse rate if they stop taking these medications during pregnancy compared to a 26% relapse rate among those who continue taking antidepressants during pregnancy. Concerns about antidepressants’ potential effects on a fetus may have been overemphasized. Kim summarized the literature on antidepressants in pregnancy, concluding that there is a preponderance of evidence that antidepressants are safe for the mother and fetus, with few serious effects having been observed. Some researchers have been concerned about risks of persistent pulmonary hypertension or autism among offspring of women who took antidepressants during pregnancy, but studies have shown that the absolute risk of either is small. Stay tuned—on Wednesday we’ll discuss a new large and comprehensive study in which most SSRIs showed no link to birth defects, but fluoxetine and paroxetine were associated with risks of certain birth defects.
Editor’s Note: For mild depression during pregnancy, exercise and psychotherapy might be optimal, along with folate and vitamin D3. For moderate depression, omega-3 fatty acids might also be helpful, but it now appears that rTMS would be less risky than electroconvulsive therapy (ECT), which in the past has been a typical recommendation for pregnant women, but which exposes the fetus to the effects of anesthesia and seizure. In her summary Kim recommended that women with a pattern of recurrent depression continue antidepressant treatment, especially since a mother’s depression itself poses non-trivial risks to the fetus.
A Note on Genetic Inheritance
Genetic inheritance is not everything, according to J. Craig Venter, pioneering genetic scientist responsible for sequencing the human genome in 2001:
“Human biology is actually far more complicated than we imagine. Everybody talks about the genes that they received from their mother and father, for this trait or the other. But in reality, those genes have very little impact on life outcomes. Our biology is far too complicated for that and deals with hundreds of thousands of independent factors. Genes are absolutely not our fate. They can give us useful information about the increased risk of a disease, but in most cases they will not determine the actual cause of the disease, or the actual incidence of somebody getting it. Most biology will come from the complex interaction of all the proteins and cells working with the environmental factors, not driven directly by the genetic code.”
Personalizing Transcranial Magnetic Stimulation for Better Results
At the 3rd Annual Meeting of the Clinical TMS Society this past May, this editor (Robert M. Post) discussed remission rates for repeated transcranial magnetic stimulation (RTMS), which at 30% leave room for improvement. It may be possible to personalize treatment parameters to achieve better success in individual patients.
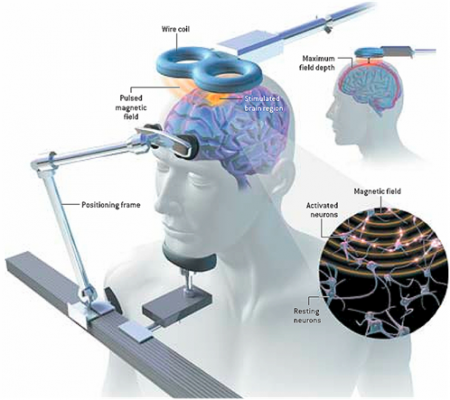
RTMS is a non-invasive procedure in which a magnetic coil placed near a patient’s head delivers electrical currents to their brain. High frequency stimulation (10Hz to 20Hz) increases brain activity as measured on PET scans, and low frequency stimulation (1Hz) decreases it, so it might be possible to choose the best frequency for an individual patient based on their baseline level of brain activity. If this is not possible, patients who fail to respond to one frequency might be switched over to the other frequency.
RTMS can be given during active positive cognitive behavioral therapy. Patients primed with positive thoughts and feelings may find that rTMS enhances these. (Some practitioners in the audience indicated that they already combine therapy and rTMS and have seen good results).
RTMS might enhance extinction learning, or the breaking of a habitual way of thinking, as it stimulates the media prefrontal cortex, a critical area for new learning. D-cycloserine, a partial glutamate agonist, has been shown to enhance extinction, and since rTMS releases glutamate and BDNF, it might also do the same.
It may also be possible to use rTMS in conjunction with medications (such as valproate) that affect epigenetic changes on the histones around which DNA is wrapped (because valproate is a histone de-acetylase inhibitor, preventing the removal of acetyl groups from the histones and allowing them to be transcribed more easily). Valproate enhances extinction learning in animals, so the stimulation of the medial prefrontal cortex and the increase in BDNF and glutamate that occur with rTMS may interact to potentiate this extinction learning.
One could also envision using rTMS to enhance learning during the memory re-consolidation window that opens 5 minutes to an hour after a person actively recalls old memories, and which can lead to more permanent memory alterations in anxiety disorders, post-traumatic stress disorder (PTSD), and substance abuse.
Since patients are awake and able to talk during rTMS, making use of evoked thoughts and memories specifically for a given patient may lead to enhanced clinical effects, because these neural pathways are more selective activated and potentiated, leading to what is called experience-dependent neuroplasticity.
Chronic Drug Use and Recovery
 George Koob, Director of the National Institute on Alcohol Abuse and Alcoholism, discussed the neuroscience of chronic drug use at the 2015 meeting of the Society of Biological Psychiatry. His basic message was that chronic drug use is associated with A) loss of the reward value of the drug and B) a progressive increase in dysphoria and stress when off the drug. Both factors drive craving and drug seeking.
George Koob, Director of the National Institute on Alcohol Abuse and Alcoholism, discussed the neuroscience of chronic drug use at the 2015 meeting of the Society of Biological Psychiatry. His basic message was that chronic drug use is associated with A) loss of the reward value of the drug and B) a progressive increase in dysphoria and stress when off the drug. Both factors drive craving and drug seeking.
Access to high as opposed to moderate doses of a drug lead to an escalation in drug intake, and associated persistent increases in withdrawal dysphoria, which Koob called “the dark side.”
Koob explained that a month of detoxification is not sufficient, and that people quitting a drug need more time to let dopamine increase and to let levels of corticotropin releasing factor (CRF), which drives the anxiety and dysphoria of withdrawal, normalize. He stressed that for people addicted to opiates, it is important to taper levels of the drug to minimize withdrawal symptoms.
In addition to CRF, dynorphin also plays a role in chronic drug abuse. This opiate peptide acts at kappa opiate receptors and is associated with anxiety, dysphoria, and psychosis as opposed to morphine, which acts at mu opiate receptors and is associated with euphoria and decreased pain. Koob found that administration of the kappa opiate antagonist norbinaltorphimine (nor-BNI) blocks dose escalation of methamphetamine and brings abstinence-related compulsive drug seeking back to baseline.
Chronic Stress Makes You Stupid
At the 2015 meeting of the Society of Biological Psychiatry, Bruce McEwen, professor of neuroscience at Rockefeller University, gave an overview of stress’s effect on the brain. He explained that “chronic stress makes you stupid,” and said that while one can compensate for the effects of chronic stress, one cannot reverse them.
Short-term stress can be helpful, increasing cortisol, with generally positive effects. When stress lasts longer, the chronic increase in cortisol starts to cause problems: impaired memory, endangered neurons, decreased bone and muscle, and metabolic abnormalities.
McEwen said that chronic stress shrinks the dendrites in the medial prefrontal cortex and the hippocampus. A healthy prefrontal cortex is necessary for new learning and memory.
Chronic stress also enlarges the orbital frontal cortex and the amygdala. An oversized orbital frontal cortex can induce habits, making a person susceptible to repetitive thinking and obsessions, addictions, or other compulsive behavior. An oversized amygdala can provoke fear and anxiety.
Editor’s Note: Stress can make people more susceptible to substance abuse, which in turn leads to losses in cortical control.
Another Expert Opinion on PTSD Treatment: Prazosin, SSRIs, and Mirtazapine
Researcher Albert Sattin has had extensive experience treating veterans with post-traumatic stress disorder (PTSD) in the U.S. Department of Veteran’s Affairs medical system. He believes that what is known as “treatment resistance” is really under-treatment, and he described his recommended regimen for thorough treatment of PTSD to this editor (Robert M. Post) at the 2015 meeting of the Society of Biological Psychiatry in May.
One of the key elements in the regimens he prescribes for patients with PTSD is prazosin, an alpha-1 antagonist drug used to treat hypertension. Extensive placebo-controlled data by another researcher, Murray Raskind, and colleagues supports the use of prazosin in PTSD. It is typically used to prevent nightmares, but Raskind, Sattin, and others find it has a broad range of positive effects in most domains of PTSD.
Sattin’s key insight is that prazosin should be administered three times a day because of its short half-life. This allows for the treatment of daytime as well as sleep-related PTSD symptoms. Sattin has patients choose from three schedules: 6am, 2pm, 10pm; 7am, 3pm, 11pm; or 8am, 4pm, 12am. Prazosin comes in 1mg, 2mg, and 5mg tablets, but patients must begin by taking the 1mg doses to reduce the risk of orthostatic hypotension (low blood pressure upon standing up), slowly increasing the dose as tolerated and if needed for symptom improvement.
Raskind reported in a poster at the same meeting that in a study of active duty combat soldiers, elevated blood pressure at baseline predicted that a patient would respond well to prazosin, so for patients with elevated blood pressure, Sattin starts with prazosin.
For other patients, Sattin begins by prescribing one of the two selective serotonin reuptake inhibitors (SSRIs) approved by the Federal Drug Administration for use in PTSD—sertraline (Zoloft) or paroxetine (Paxil)—and then adds the antidepressant mirtazapine (Remeron) if necessary. If the patient still remains symptomatic, Sattin then adds prazosin to the regimen with the added warning to take time sitting up or standing up to avoid potential dizziness from low blood pressure. Read more
Mindfulness Interventions May Reduce Substance Use and Cravings
A 2014 meta-analysis of the literature to date on mindfulness-based interventions (MBIs) for substance use disorders suggests that these interventions can reduce consumption of alcohol, cocaine, amphetamines, marijuana, cigarettes, and opiates, compared to several types of controls. The research by Alberto Chiesa and Alessandro Serretti, published in the journal Substance Use and Misuse, includes 24 studies published before 2012. The authors also found some evidence that MBIs are associated with reduced craving and increased mindfulness. Most of the studies included in the meta-analysis were small, so their generalizability is limited.
A 2014 article by S. Bowen and colleagues in the journal JAMA Psychiatry compared mindfulness-based relapse prevention with standard relapse prevention and treatment as usual for people recovering from substance abuse. Mindfulness-based relapse prevention combines the cognitive behavioral approach of standard relapse prevention with MBIs that have been successful in other studies.
Bowen et al. found that both standard relapse prevention and mindfulness-based relapse prevention lowered the risk of relapse and reduced days of substance use at 6 months, compared to treatment as usual. The standard treatment delayed first drug use, but the mindfulness intervention decreased use at the 12-month mark compared to both standard relapse prevention and treatment as usual.
Substance Abuse Is a Treatable Brain Disorder
Depression and bipolar disorder come with a high incidence of substance abuse. It is important to realize that there are now good medicines to treat the addictions as well as the primary mood disorders they accompany. At the 2015 meeting of the American Psychiatric Association, Nora Volkow, director of the National Institute of Drug Abuse, encouraged psychiatrists to think of addiction as a “disease of the brain that disrupts the systems that allow people to exert self-control,” saying that this would help reduce stigma both for insurance companies and for the wider public.
Most treatment of substance abuse in bipolar disorder is off-label. Doctors must infer indirect evidence of the possible efficacy of each drug in bipolar disorder from studies in those with only the primary addiction, for example, cocaine abuse without bipolar disorder.
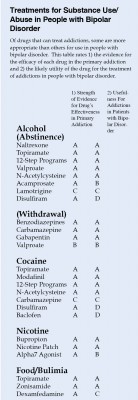
The table at right is a preliminary rating of 1) the strength of the evidence for the efficacy of each drug in the primary addiction and 2) the likely utility of the drug for the treatment of addictions in people with bipolar disorder. For example, the drug baclofen has excellent evidence of efficacy in cocaine addiction, but gets a D for utility in bipolar disorder because baclofen can exacerbate depression.
This list is provisional, and the subjective grades for each drug are likely to change as more research is collected on these treatments. Consult a doctor if you are seeking treatment for bipolar disorder and/or substance abuse.