THE PATHOPHYSIOLOGY OF SCHIZOPHRENIA IS BECOMING CLEARER
David Lewis of U. Pittsburgh showed that the glutamate neurons in the prefrontal cortex of patients with schizophrenia are deficient in the gamma (30-50 Hz) oscillations that are responsible for normal working memory.
Not only are dendrites and spines deficient in these neurons in layer 3 of the cortex, but there is a deficit in parvalbumin GABA inhibitory neurons. The GABA enzyme GAD 67 is lower, producing less inhibition. The frontal neurons are hypoactive and there is less BDNF and oxidative phosphorylation present, yielding decreases in mitochondrial function.
Scientific Mechanisms of Rapid-Acting Antidepressants
A pyramidal cell (Photo by Bob Jacobs, Laboratory of Quantitative Neuromorphology Department of Psychology Colorado College)
At a recent symposium, researcher Francis McMahon provided electrophysiological evidence that several different types of rapid-acting antidepressants—low-dose ketamine, scopolamine, and rapastinel (a partial agonist of the neurotransmitter NMDA)—act by decreasing the inhibitory effects of GABAergic interneurons on excitatory neurons called pyramidal cells, thus increasing synaptic firing.
Researcher Ronald Duman further dissected these effects, showing that ketamine and its active metabolite norketamine reduce the steady firing rate of GABA interneurons by blocking NMDA receptors, while the partial agonist rapastinel acts on the glutamate neurons directly, and both increase the effects of a type of glutamate receptors known as AMPA. These effects were demonstrated using a virus to selectively knock out GluN2B glutamate receptor subunits in either GABA interneurons or glutamate neurons.
Increasing AMPA activity increases synapse number and function and also increases network connectivity, which can reverse the effects of stress. Duman and colleagues further showed that when light is used to modulate pyramidal cells (a process called optogenetic stimulation) in the medial prefrontal cortex, different effects could be produced. Stimulating medial prefrontal cortex cells that contained dopamine D1 receptors, but not D2 receptors, produced rapid and sustained antidepressant effects. Conversely, inhibiting these neurons blocked the antidepressant effects of ketamine. Stimulating the terminals of these D1-containing neurons in the basolateral nucleus of the amygdala was sufficient to reproduce the antidepressant effects. These data suggest that stimulation of glutamate D1 pyramidal neurons from the medial prefrontal cortex to the basolateral nucleus of the amygdala is both necessary and sufficient to produce the antidepressant effects seen with ketamine treatment.
Researcher Hailan Hu reported that NMDA glutamate receptors drive the burst firing of lateral habenula (LHb) neurons, which make up the depressogenic or “anti-reward center” of the brain and appear to mediate anhedonic behavior (loss of interest or enjoyment) in animal models of depression. Ketamine blocks the burst firing of the LHb neurons, which disinhibits monoamine reward centers, enabling ketamine’s rapid-onset antidepressant effects. This may occur because inhibitory metabotropic glutamate receptors (mGluR-2) are activated, decreasing the release of glutamate.
MGluR-2 may also help explain the antidepressant effects of acetyl-L-carnitine supplements. L-carnitine is an amino acid that is low in the blood of depressed patients. The supplement acetyl-L-carnitine (ACL) activates the DNA promoter for mGluR-2, increasing its production and thus decreasing excess glutamate release. The acetyl group of the ACL binds to the DNA promoter for mGluR-2, thus this process seems to be epigenetic. Epigenetic mechanisms affect the structure of DNA and can be passed on to offspring even though they are not encoded in the DNA’s genetic sequence.
Supplement Acetyl-L-Carnitine May Treat Stress and Depression
N-acetylcysteine (NAC), an antioxidant sold in health food stores, has several beneficial effects on brain and behavior. It improves depression and can reduce cravings for cocaine, alcohol, marijuana, and nicotine, and can also help control habit-driven behaviors such as gambling, compulsive hair-pulling, and symptoms of obsessive-compulsive disorder (OCD).
New research, particularly by researcher Nascaa and colleagues in 2014 and 2016, has identified a related compound, acetyl-l-carnitine (ALC), as an anti-stressor and antidepressant in animals, and researchers have begun to explore its use in people. ALC has been found to improve mitochondrial function and improve recovery from peripheral nerve damage. ALC also inhibits the release of glutamate, which can prevent depressive behaviors following stress.
A 2004 study by P. Ruggenenti and colleagues in the journal Hypertension found that in people, 1 gm of ALC taken twice daily safely improved arterial hypertension, insulin resistance, impaired glucose tolerance, and low levels of adiponectin in the blood (a risk factor for diabetes) in subjects at increased cardiovascular risk.
In a 2014 article in the Journal of Psychiatric Research, researcher S.M. Wang and colleagues reviewed evidence that ALC improves mild depression. Two randomized clinical trials indicated that ALC was more effective than placebo for mild depression. Two other randomized clinical trials showed that ALC was as effective as the antidepressants fluoxetine and amisulpride for mild depression. The supplement was as tolerable as placebo and better tolerated than fluoxetine and amisulpride. Wang and colleagues suggested that more clinical trials are needed to confirm that ALC is effective in depression.
Editor’s Note: If further clinical trials confirm the antidepressant effects of ALC, it could represent a new way to treat chronic stress and depression and regulate insulin. Together these effects could reduce the cardiovascular risks that accompany depression.
Medications that Regulate Glutamate Transporters Can Reduce Cocaine Craving
Glia are brain cells that surround neurons and synapses, protecting and insulating them. Chronic cocaine use and withdrawal changes the way certain glial cells, called astrocytes, interact with neurons. In particular, chronic cocaine use and withdrawal can shrink astrocytes and cause them to pull away from neurons. Cocaine use and withdrawal also interfere with the way the neurotransmitter glutamate is cleared from synapses and transported into astrocytes.
New research shows that certain medications that regulate and increase the movement of glutamate from the synapse into glial cells can reduce cravings for cocaine.
In studies of rats chronically exposed to cocaine and then denied access to it, treatment with these glutamate-targeting medications reduces the rats’ cocaine-seeking behaviors. The medications include N-acetylcysteine (NAC), an antioxidant that can reduce habitual behaviors, including addictive behaviors; riluzole, a treatment for amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease; the antibiotic ceftriaxone; and propentofylline, which has been explored as a possible treatment for dementia and stroke.
RTMS Study Identifies Glutamate as a Biomarker for Depression Treatment
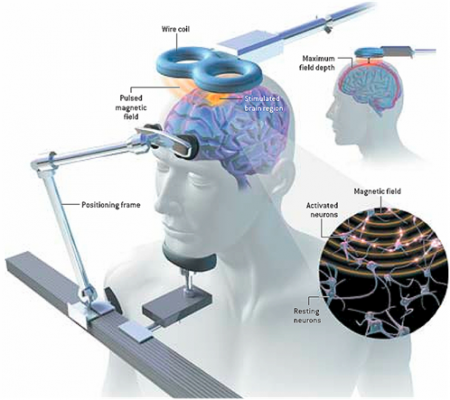 At the 3rd Annual Meeting of the Transcranial Magnetic Stimulation Society, Canadian researcher Frank MacMaster discussed his study of repeated transcranial magnetic stimulation (rTMS) in 50 children with depression. RTMS is a non-invasive procedure in which an electromagnetic coil is placed against the side of the forehead and magnetic pulses that can penetrate the scalp are converted into small electrical currents that stimulate neurons in the brain. The study was designed to identify biomarkers, or characteristics that might indicate which patients were likely to respond to the treatment. All of the patients received rTMS at a frequency of 10 Hz. Using magnetic resonance spectroscopy (MRS) technology, MacMaster found that children who responded well to rTMS treatment had low levels of the neurotransmitter glutamate at the beginning of the study, but their glutamate levels increased as their depression improved. Children who didn’t improve had higher glutamate levels at the beginning of the study, and these fell during the rTMS treatment.
At the 3rd Annual Meeting of the Transcranial Magnetic Stimulation Society, Canadian researcher Frank MacMaster discussed his study of repeated transcranial magnetic stimulation (rTMS) in 50 children with depression. RTMS is a non-invasive procedure in which an electromagnetic coil is placed against the side of the forehead and magnetic pulses that can penetrate the scalp are converted into small electrical currents that stimulate neurons in the brain. The study was designed to identify biomarkers, or characteristics that might indicate which patients were likely to respond to the treatment. All of the patients received rTMS at a frequency of 10 Hz. Using magnetic resonance spectroscopy (MRS) technology, MacMaster found that children who responded well to rTMS treatment had low levels of the neurotransmitter glutamate at the beginning of the study, but their glutamate levels increased as their depression improved. Children who didn’t improve had higher glutamate levels at the beginning of the study, and these fell during the rTMS treatment.
MacMaster hopes that glutamate levels and other biological indicators such as inflammation will eventually pinpoint which treatments are likely to work best for children with depression. At the meeting, MacMaster said that in Canada, only a quarter of the 1,200,000 children with depression receive appropriate treatment for it. Very little funding is devoted to research on children’s mental health, a serious deficit when one considers that most depression, anxiety, attention deficit hyperactivity disorder (ADHD), bipolar disorder, oppositional behavior, conduct disorder, and substance abuse begins in childhood and adolescence, and early onset of these illnesses has been repeatedly linked to poorer outcomes.
Editor’s Note: The strategy of identifying biomarkers is an important one. MacMaster noted that this type of research is possible due to the phenomenal improvements in brain imaging techniques that have occurred over the past several decades. These techniques include magnetic resonance imaging (MRI) to a resolution of 1 mm; functional MRI; diffusion tensor imaging (DTI), which can depict the connectivity of white matter tracts; and spectroscopy, which can be used to identify chemical markers of neuronal health and inhibitory and excitatory neurotransmitters, and analyze membrane integrity and metabolic changes. These methods provide exquisite views of the living brain, the most complicated structure in the universe. The biomarkers these techniques may identify will allow clinicians to predict how a patient will respond to a given treatment, to choose treatments more rapidly, and to treat patients more effectively.
Neurosteroid Allopregnanolone May Improve Bipolar Depression
Sherman Brown of the University of Texas Southwestern reports that the neurosteroid allopregnanolone has positive effects in bipolar depression. Patients in Brown’s study received doses of 100mg capsules twice daily during the first week, then one capsule in the morning and two capsules in the evening during the second week, and two capsules in the morning and three capsules in the evening during the third week.
Neurosteroids can change the excitability of neurons through their interactions with the neurotransmitters that carry signals from neurons across synapses. Among the various types of neurotransmitters, GABA plays an inhibitory role, while glutamate is responsible for excitability. Allopregnanolone, which is naturally produced in the body, has positive effects on GABA receptors and inhibitory effects on glutamate NMDA receptors, so that it increases the balance of inhibition (GABA) over excitation (glutamate).
How Inflammation Increases Glutamate Overexcitation And Neurotoxicity
Research has shown a link between inflammation and mental illness. Inflammation leads to a series of chemical changes that can overexcite neurons and interfere with the protection of neurons.
Inflammation increases the production of indoleamine-pyrrole 2,3-dioxygenase (IDO), an enzyme that breaks down the amino acid tryptophan into kynurenic acid and quinolinic acid. They in turn increase glutamate, the main excitatory neurotransmitter, and decrease brain-derived neurotrophic factor (BDNF), which keeps neurons healthy.
Kynurenic acid stimulates microglia, which clean up the central nervous system as a form of immune defense, to produce inflammatory cytokine proteins.
Quinolinic acid directly stimulates glutamate receptors and encourages glutamate release from astrocytes. Quinolinic acid also blocks glutamate removal that would normally occur through reuptake into the astrocytes, leading to more stimulation of extrasynaptic glutamate receptors and decreases in BDNF.
Quinolinic acid’s effects are opposite to those of the antidepressant ketamine, which blocks glutamate NMDA receptors and increases BDNF. When people are given interferon protein for the treatment of cancers, quinolinic acid increases in cerebrospinal fluid, inducing depression. The severity of depression induced is correlated with the patient’s levels of quinolinic acid.
It appears that ketamine has indirect anti-inflammatory effects through its ability to block glutamate receptors and increase BDNF.
Another Blocker of Glutamate Receptor Function with Rapid Antidepressant Effects
Certain drugs such as ketamine and memantine that work by blocking activity at the NMDA receptor for the excitatory neurotransmitter glutamate have antidepressant effects. D-cycloserine is a drug that has a related mechanism and is being studied as an antidepressant. At high doses the drug acts as an antagonist at the glycine site of the NMDA receptor, blocking glycine’s ability to facilitate glutamate transmission through the receptor.
Joshua Kantrowitz, a researcher at Columbia University, reported at a recent scientific meeting that the rapid-onset antidepressant effects of D-cycloserine could be maintained for eight weeks. Similar findings were published in the Archives of General Psychiatry in 2010 and were reported in another study by Uriel Heresco-Levy in a 2013 article in the Journal of Neuropsychopharmacology.
Glutamate is the major excitatory neurotransmitter in the brain and is important for the development of long-term memory. However, glutamate overactivity may contribute to depression. Decreasing this overactivity (with ketamine, memantine, or D-cycloserine) may produce antidepressant effects.
Treating Substance Abuse: A Step Forward
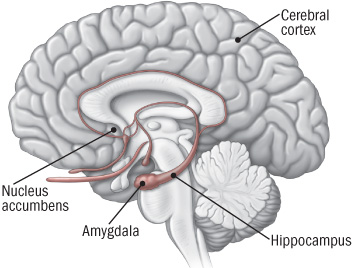 New discoveries in neuroanatomy are helping clarify what addiction looks like in the brain. Peter Kalivas of the Medical University of South Carolina reported at the 2013 meeting of the Society of Biological Psychiatry that most drugs of abuse alter glutamate levels and the plasticity of synapses in the nucleus accumbens, the reward area of the brain. Glutamate is the main excitatory neurotransmitter in the brain, and compulsive habits may be associated with increased release of glutamate in this brain area.
New discoveries in neuroanatomy are helping clarify what addiction looks like in the brain. Peter Kalivas of the Medical University of South Carolina reported at the 2013 meeting of the Society of Biological Psychiatry that most drugs of abuse alter glutamate levels and the plasticity of synapses in the nucleus accumbens, the reward area of the brain. Glutamate is the main excitatory neurotransmitter in the brain, and compulsive habits may be associated with increased release of glutamate in this brain area.
During chronic cocaine administration, for example, the neurons in the nucleus accumbens lose their adaptive flexibility and their ability to respond to signals from the prefrontal cortex. Normally, low levels of stimulation would induce long-term depression (LTD) while high levels of stimulation would induce long-term potentiation (LTP). These are long-term changes in the strength of a synapse, which allow the brain to change with learning and memory. When long-term potentiation and long-term depression are no longer possible, memory and new learning in response to messages from the prefrontal cortex are diminished.
Given this absence of flexible responding, animals extinguished from cocaine self-administration (when a lever they had pressed to receive cocaine ceases to provide cocaine) are highly susceptible to cocaine reinstatement if a stressor is presented or if a signal appears that suggests the availability of cocaine. This cocaine reinstatement is associated with high levels of glutamate in the nucleus accumbens, so Kalivas reasoned accurately that lowering these levels would be associated with a lesser likelihood of cocaine reinstatement.
The drug N-acetylcysteine (NAC), which is available from health food stores, decreases the amount of glutamate in the nucleus accumbens by inducing a glutamate transporter in glial cells that helps clear excess synaptic glutamate. In Kalivas’ research, NAC prevented cocaine reinstatement, cocaine-induced anatomical changes in spine shape (bigger, stubby spines), and the loss of long-term potentiation and long-term depression in the nucleus accumbens.
The findings on NAC in animal studies led to a series of important small placebo-controlled clinical trials in people with a variety of addictions, and positive results have been found using NAC in people addicted to opiates, cocaine, alcohol, marijuana, and gambling. It also decreases hair-pulling in trichotillomania and reduces stereotypy and irritability in children with autism.
NAC also appears to be effective in the treatment of unipolar and bipolar depressed patients in placebo-controlled trials by Australian researcher Michael Berk. Thus, NAC could be useful for patients with affective disorders who are also having difficulties with comorbid substance use.
Some antibiotics (that are not commonly available) also induce the glutamate transporter and glial cells of the nucleus accumbens, offering a potential new approach to treating some addictions.
New Research on Ketamine
 The drug ketamine can produce antidepressant effects within hours when administered intravenously.
The drug ketamine can produce antidepressant effects within hours when administered intravenously.
Finding an Appropriate Control
Comparing ketamine to placebo has challenges because ketamine produces mild dissociative effects (such as a feeling of distance from reality) that are noticeable to patients. At the 2013 meeting of the Society of Biological Psychiatry, James W. Murrough and collaborators at the Mount Sinai School of Medicine reported their findings from the first controlled trial of intravenous ketamine in depression that uses an active control, the short-acting benzodiazepine midazolam, which has sedative effects and decreases anxiety, but is not known as an antidepressant. On virtually all measures intravenous ketamine was a more effective antidepressant following 2 infusions per week.
These data help dispel one of the criticisms of intravenous ketamine, that studies of the drug have not been sufficiently blinded (when patients and medical staff are kept from knowing which patients receive an active treatment and which are in the placebo control group) and that the lack of an appropriate active placebo contributed to the dramatic findings about ketamine’s antidepressant effects. It now appears that these criticisms have been appropriately answered and that intravenous ketamine is highly effective not only in comparison to placebo but also to an active comparator.
This research was presented as a poster at the meeting and published as abstract #442 in the meeting supplement to the journal Biological Psychiatry, Volume 73, Number 9S, and was also published in the Archives of General Psychiatry in 2013.
Slowing Down Ketamine Infusions to Reduce Side Effects
Ketamine is commonly given in 40-minute intravenous infusions. Timothy Lineberry from the Mayo Clinic reported in Abstract #313 from the meeting that slower infusions of ketamine over 100 minutes were also effective in producing antidepressant effects in patients with treatment-resistant depression. Lineberry’s research group used the slower infusion in order to increase safety and decrease side effects, such as the dissociative effects discussed above. In the 10 patients the group studied, they observed a response rate of 80% and a remission rate of 50% (similar to ketamine’s effects with 40-minute infusions).
Family or Personal History of Alcohol Dependence Predicts Positive Response to Ketamine in Depression
Mark J. Niciu and collaborators at the NIMH reported in Abstract #326 that a personal or family history of alcohol dependence predicted a positive response to IV ketamine in patients with unipolar depression.
Ketamine Acts on Monoamines in Addition to Glutamate
Ketamine’s primary action in the nervous system is to block glutamate NMDA receptors in the brain. In addition to its effects on glutamate, it may also affect the monoamines norepinephrine and dopamine. Kareem S. El Iskandarani et al. reported in Abstract #333 that in a study of rats, ketamine increased the firing rate of norepinephrine neurons in a part of the brain called the locus coeruleus and also increased the number of spontaneous firing dopamine cells in the ventral tegmental area of the brain.
Editor’s Note: These data showing that ketamine increased the activity of two monoamines could help explain ketamine’s ability to induce rapid onset of antidepressant effects, in addition to its ability to immediately increase brain-derived neurotrophic factor (BDNF, important for long-term memory and the creation of new synapses) and to restore healthy mushroom-shaped spines on the dendrites of neurons in the prefrontal cortex.