In Animal Model, Long-Term THC Exposure Interferes with Cortical Control of the Nucleus Accumbens

In an article in the journal Biological Psychiatry, researchers Eun-Kyung Hwang and Carl R. Lupica reported that in rats, long-term use of THC (tetrahydrocannabinol) weakens input from the cortex to the reward area of the brain, the nucleus accumbens (NAc). Long-term THC use also strengthens connections to the NAc from emotional control (limbic) regions, such as the basolateral amygdala and ventral hippocampus. Hwang and Lupica reason that this shift from cortical control of the NAc to limbic control likely contributes to the cognitive and psychiatric symptoms associated with cannabis use.
Editor’s Note: Street marijuana largely contains THC rather than CBD, the beneficial, anxiety-reducing component of cannabis. Cannabis products are being decriminalized, but it is important to remember that those with THC are linked to cannabis use disorder and increased susceptibility to psychiatric illness. Patients with bipolar disorder who use marijuana also have a more adverse course of illness than those who do not use it.
Thalamus Implicated in Depression-Like Behavior and Resilience to It
At the 2014 meeting of the International College of Neuropsychopharmacology, researcher Scott Russo described characteristics of rodents who showed depression-like behavior after 10 days of exposure to a larger, more aggressive animal (a phenomenon known as defeat stress). These animals exhibited many behaviors that resembled human depression, including anxiety-like behaviors while navigating a maze; activation of the hypothalamic-pituitary-adrenal axis; circadian rhythm abnormalities; metabolic changes such as glucose intolerance; susceptibility to addiction; anhedonia, a lack of interest in sucrose, sex or intracranial self-stimulation; and profound and permanent social avoidance.
In susceptible animals, Russo found anatomical changes in the GABAergic neurons of the nucleus accumbens (also known as the ventral striatum), including increased numbers of synapses and a greater number of stubby spines on dendrites (the branched projections of neurons where electrical signals are passed from one cell to the next), as well as greater excitability of glutamatergic input, observed as excitatory post-synaptic potentials.
Russo’s attempt to identify these key neurons among the billions of neurons and the 100 to 500 trillion synapses in the brain was like the search for a needle in a haystack, but thinks he found it. The medium spiny neurons of the nucleus accumbens contain GABA and receive synapses from the prefrontal cortex, amygdala, and intralaminar nucleus of the thalamus (ILT), in addition to dopamine inputs from the VTA, and cholinergic, somatostatin, and orexin inputs. Russo found that it was the ILT inputs that conveyed susceptibility to defeat stress, and their presynaptic endings showed increased levels of glutamate transporters (VGLUT-2). Driving the ILT was sufficient to cause the rodents to display the depression-like behaviors, and silencing the ILT during defeat stress prevented the susceptible behaviors (like social avoidance) and promoted resilience.
The Nucleus Accumbens in Depression
Brain-derived neurotrophic factor (BDNF) keeps neurons healthy and is critical for long-term memory and synapse formation. BDNF levels increase in the nucleus accumbens (the brain’s reward center) and decrease in the hippocampus during clinical depression and chronic cocaine use. In rodents, the same changes in BDNF levels occur during defeat stress (which resembles human depression).
Rodents who are repeatedly defeated by a larger rodent exhibit behaviors such as social withdrawal, lethargy, and decreased interest in sucrose. The increases in BDNF in the nucleus accumbens of these rodents could reflect the learning that takes place during the repeated defeat stress and the depression-like behaviors that follow it. Blocking the BDNF increases in the nucleus accumbens prevents these behaviors from developing.
Chadi Abdallah and other researchers at Yale University recently found that the left nucleus accumbens of patients with treatment-resistant depression is enlarged compared to normal controls, and the drug ketamine, which produces rapid-onset antidepressant effects, rapidly decreases the volume of the nucleus accumbens in the depressed patients. The mechanism by which it does so is unknown, but could reflect some suppression of the depressive learning.
Any relationship between the volume of the nucleus accumbens and its levels of BDNF is unknown, but ketamine’s effect on the size of this brain region could be linked to a decrease in the defeat-stress memories.
Treating Substance Abuse: A Step Forward
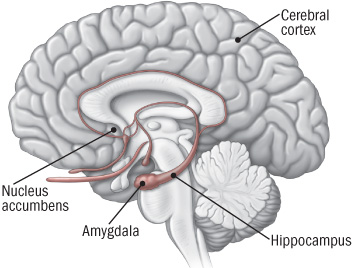 New discoveries in neuroanatomy are helping clarify what addiction looks like in the brain. Peter Kalivas of the Medical University of South Carolina reported at the 2013 meeting of the Society of Biological Psychiatry that most drugs of abuse alter glutamate levels and the plasticity of synapses in the nucleus accumbens, the reward area of the brain. Glutamate is the main excitatory neurotransmitter in the brain, and compulsive habits may be associated with increased release of glutamate in this brain area.
New discoveries in neuroanatomy are helping clarify what addiction looks like in the brain. Peter Kalivas of the Medical University of South Carolina reported at the 2013 meeting of the Society of Biological Psychiatry that most drugs of abuse alter glutamate levels and the plasticity of synapses in the nucleus accumbens, the reward area of the brain. Glutamate is the main excitatory neurotransmitter in the brain, and compulsive habits may be associated with increased release of glutamate in this brain area.
During chronic cocaine administration, for example, the neurons in the nucleus accumbens lose their adaptive flexibility and their ability to respond to signals from the prefrontal cortex. Normally, low levels of stimulation would induce long-term depression (LTD) while high levels of stimulation would induce long-term potentiation (LTP). These are long-term changes in the strength of a synapse, which allow the brain to change with learning and memory. When long-term potentiation and long-term depression are no longer possible, memory and new learning in response to messages from the prefrontal cortex are diminished.
Given this absence of flexible responding, animals extinguished from cocaine self-administration (when a lever they had pressed to receive cocaine ceases to provide cocaine) are highly susceptible to cocaine reinstatement if a stressor is presented or if a signal appears that suggests the availability of cocaine. This cocaine reinstatement is associated with high levels of glutamate in the nucleus accumbens, so Kalivas reasoned accurately that lowering these levels would be associated with a lesser likelihood of cocaine reinstatement.
The drug N-acetylcysteine (NAC), which is available from health food stores, decreases the amount of glutamate in the nucleus accumbens by inducing a glutamate transporter in glial cells that helps clear excess synaptic glutamate. In Kalivas’ research, NAC prevented cocaine reinstatement, cocaine-induced anatomical changes in spine shape (bigger, stubby spines), and the loss of long-term potentiation and long-term depression in the nucleus accumbens.
The findings on NAC in animal studies led to a series of important small placebo-controlled clinical trials in people with a variety of addictions, and positive results have been found using NAC in people addicted to opiates, cocaine, alcohol, marijuana, and gambling. It also decreases hair-pulling in trichotillomania and reduces stereotypy and irritability in children with autism.
NAC also appears to be effective in the treatment of unipolar and bipolar depressed patients in placebo-controlled trials by Australian researcher Michael Berk. Thus, NAC could be useful for patients with affective disorders who are also having difficulties with comorbid substance use.
Some antibiotics (that are not commonly available) also induce the glutamate transporter and glial cells of the nucleus accumbens, offering a potential new approach to treating some addictions.
How Illness Progresses In The Recurrent Affective Disorders
This editor (RM Post) in collaboration with Jacqueline Fleming and Flavio Kapczinski published the article “Neurobiological mechanisms of illness progression in the recurrent affective disorders” in the Journal of Psychiatric Research this year. The article built on several themes about the progression of bipolar illness that had been explored in previous research.
These themes include:
- The likely acceleration of repeated episodes as a function of the number of prior episodes (episode sensitization)
- The increased responsivity of the illness to repeated stressors (stress sensitization)
- The increased behavioral reactivity to repeated use of psychomotor stimulants such as cocaine (stimulant-induced behavioral sensitization)
Not only are these observations well documented in the scientific literature, but recent observations also suggest that each type of sensitization can show cross-sensitization to the other two types. That is, individuals exposed to repeated stressors are more likely both to experience affective illness episodes and to adopt comorbid substance abuse. In a similar way, episodes of an affective disorder and stressors may also be associated with the relapse into drug administration in those who have been abstinent.
In addition to these mechanisms of illness progression in the recurrent affective disorders, the new article reviews the literature showing that the number of affective episodes or the duration of the illness appear to be associated with a variety of other clinical and neurobiological variables.
The number of affective episodes a patient experiences is associated with the degree of cognitive dysfunction present in their bipolar illness, and experiencing more than 4 episodes of unipolar or bipolar depression is a risk factor for dementia in late life. A relative lack of response to most treatments is also correlated with the number of prior episodes, and this holds true for response to naturalistic treatment in general. While most of these data are correlational and the direction of causality cannot be ascertained for certain, it is likely that the number of affective episodes and/or their duration could account for and drive difficulties with treatment and with cognitive function.
If this were the case, one would expect to see a variety of neurobiological correlates with the number of prior episodes or duration of illness, and in the article we summarize those that have been found in unipolar and bipolar disorder. Considerable data indicate that cortical volume and degrees of prefrontal cortical dysfunction can vary as a function of number of prior episodes. There is evidence that increased activity of the amygdala and the nucleus accumbens are also related to episodes or duration of illness. In those with unipolar depression, the volume of the hippocampus is decreased with longer duration of illness. Read more