Lithium Responders and Non-Responders Have Different Neuron Characteristics
After hyperpolarization (via Wikimedia Commons)
A 2017 study in the journal Molecular Psychiatry suggests that by observing the neurons of a person with bipolar disorder, you can predict whether they will respond to lithium treatment. The drug is effective in approximately 30% of those to whom it is prescribed.
Researchers led by Shani Stern and Renata Santos used stem cell research to analyze neurons from people with bipolar disorder and healthy controls.
People with bipolar disorder shared some neuron features, namely a large, fast after-hyperpolarization (a phase in which the cell’s membrane changes), which is followed by a resting period before the neuron can fire again. The large, fast hyperpolarization in people with bipolar disorder speeds up this cycle, leading to fast and sustained neuron firing. This replicated previous findings by the same researchers, which found that people with bipolar disorder are more sensitive to stimuli. In people with bipolar disorder, the threshold for a neuron to fire drops with each subsequent after-hyperpolarization.
Chronic lithium treatment reduced this hyperexcitability in some patients—and these were the patients who had a good response to lithium treatment.
Among the study participants with bipolar disorder, there were differences in the neuron profiles of those who responded well to lithium versus those who did not.
Stern and colleagues programmed a computer to recognize the electrophysiological features of neurons from lithium responders and non-responders. The computer could then analyze the neurons of a patient whose response to lithium was unknown and predict with a greater than 92% success rate whether that patient had responded well to lithium treatment.
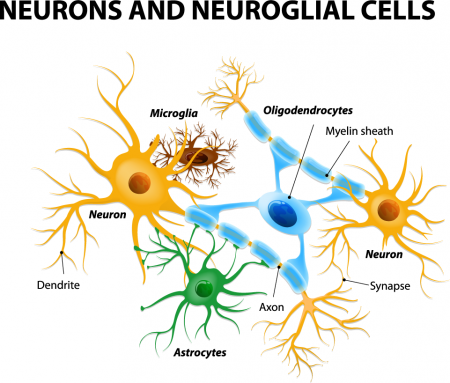
Astrocytes Can Turn Toxic
Astrocytes usually play a helpful role in the brain. These glial cells facilitate neural connections and prune unnecessary ones. However, new research details how infection or trauma can render astrocytes toxic, leading to brain disorders. In a 2017 article in the journal Nature, researcher Shane A. Liddelow and colleagues describe how resting astrocytes can become harmful reactive astrocytes.
The researchers determined that reactive astrocytes are found in brain tissues following brain injuries, or in neurological disorders including Alzheimer’s, Parkinson’s, Huntington’s, amyotrophic lateral sclerosis (ALS), and multiple sclerosis. Liddelow and colleagues also determined that microglia play a role in transforming resting astrocytes into a subtype of harmful reactive astrocytes, dubbed A1 astrocytes, and that the latter secrete a neuron-killing toxin.
A1 astrocytes lose their ability to promote neuronal survival and to create new synapses. The researchers showed that blocking the formation of A1 astrocytes prevented certain neuron deaths, and they hope this finding will lead to new treatments for brain injuries and neurological disorders.
Editor’s Note: It is possible that A1 astrocytes are also induced in patients with bipolar disorder, post-traumatic stress disorder (PTSD), and schizophrenia, as well as head traumas and neurological disorders.
Memory Activates Epigenetic Changes in Mice Brain Cells
In a 2015 article in Nature Neuroscience, Stefan Bonn and André Fischer reported that when mice were prompted to use their long-term memory to recognize a specific environment, epigenetic changes occurred in their neurons and glia. Epigenetic changes refer to chemical alterations in DNA or histones (which give DNA structure) that increase or decrease the expression of certain genes. Sometimes environmental factors lead to a methyl or acetyl group joining a strand of DNA or histones, changing how easily the genes are turned on or off.
When the mice used their long-term memory, the main change that occurred was DNA methylation in their neurons. There were also changes to histones that were linked to memory acquisition but resulted in few changes in gene expression. The DNA methylation changes, on the other hand, changed neural pathways, leading to “rewiring” of the brain.
Output From the Amygdala Mediates Reward or Fear Memories
People and animals can rapidly learn to associate environmental stimuli with positive or negative outcomes, learning what to approach or avoid as they go through daily life. The amygdala plays a role in this type of emotional learning, which can be disrupted by mood disorders. In new research, Praneeth Namburi and colleagues determined that activity at the synapses in the basolateral amygdala reveals differences in the creation of fear memories and reward memories.
In animals trained with reward and fear conditioning tasks, photostimulation of neurons that then travel from the basolateral amygdala complex to the nucleus accumbens (the brain’s reward center) is positively reinforcing, while photostimulation of neurons that will travel from the basolateral amygdala complex to the centromedial nucleus of the amygdala causes aversion. There are genetic differences between the two types of neurons, including a difference in the gene for the neurotensin-1 receptor. The researchers found that neurotensin, a neuropeptide, modulates glutamate’s effect on neurons, causing some to project to the nucleus accumbens and some to project to the centromedial nucleus of the amygdala.
The researchers wrote that the results “provide a mechanistic explanation, on both a synaptic and circuit level, for how positive and negative associations can be rapidly formed, represented, and expressed within the amygdala.”
Editor’s Note: The amygdala’s creation of opposing outputs may provide clues to the mechanisms behind mania (involving the nucleus accumbens) and depression (involving the centromedial nucleus of the amygdala).
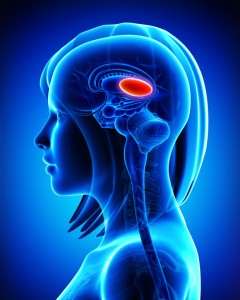
Thalamus Implicated in Depression-Like Behavior and Resilience to It
At the 2014 meeting of the International College of Neuropsychopharmacology, researcher Scott Russo described characteristics of rodents who showed depression-like behavior after 10 days of exposure to a larger, more aggressive animal (a phenomenon known as defeat stress). These animals exhibited many behaviors that resembled human depression, including anxiety-like behaviors while navigating a maze; activation of the hypothalamic-pituitary-adrenal axis; circadian rhythm abnormalities; metabolic changes such as glucose intolerance; susceptibility to addiction; anhedonia, a lack of interest in sucrose, sex or intracranial self-stimulation; and profound and permanent social avoidance.
In susceptible animals, Russo found anatomical changes in the GABAergic neurons of the nucleus accumbens (also known as the ventral striatum), including increased numbers of synapses and a greater number of stubby spines on dendrites (the branched projections of neurons where electrical signals are passed from one cell to the next), as well as greater excitability of glutamatergic input, observed as excitatory post-synaptic potentials.
Russo’s attempt to identify these key neurons among the billions of neurons and the 100 to 500 trillion synapses in the brain was like the search for a needle in a haystack, but thinks he found it. The medium spiny neurons of the nucleus accumbens contain GABA and receive synapses from the prefrontal cortex, amygdala, and intralaminar nucleus of the thalamus (ILT), in addition to dopamine inputs from the VTA, and cholinergic, somatostatin, and orexin inputs. Russo found that it was the ILT inputs that conveyed susceptibility to defeat stress, and their presynaptic endings showed increased levels of glutamate transporters (VGLUT-2). Driving the ILT was sufficient to cause the rodents to display the depression-like behaviors, and silencing the ILT during defeat stress prevented the susceptible behaviors (like social avoidance) and promoted resilience.
Mood-Stabilizing Drugs Increase Growth in Hippocampal Neurons
Lithium is known for protecting neurons by inducing neurotrophic factors and inhibiting cell death factors. In a new study, other mood-stabilizing drugs had similar neuroprotective and neurotrophic effects on cultured neurons from the hippocampus.
At the 2014 meeting of the International Society for Bipolar Disorders, CH Lee et al. presented evidence that lithium, carbamazepine, valproic acid, and lamotrigine all increase the outgrowth of dendrites from these cultured neurons. Therapeutic levels of these drugs increased the production of proteins like brain-derived neurotrophic factor (BDNF), postsynaptic density protein-95 (PSD-95), neurolignin 1 (NLG 1), beta-neurexin, and synaptophysin. However, so far only lithium has been shown to increase the volume of the human hippocampus as measured with MRI.
Residue from Nuclear Bomb Testing Shows That Contrary to Earlier Reports, Neurogenesis Occurs in the Brains of Adults
 In 2013 we reported that according to Pasco Rakic, professor of neuroanatomy at Yale University, neurogenesis (the production of new neurons) occurs only in rodents, and not in any significant amount in the brains of adult primates. However, a new carbon-dating procedure shows that the adult human brain does actually continue to create new neurons.
In 2013 we reported that according to Pasco Rakic, professor of neuroanatomy at Yale University, neurogenesis (the production of new neurons) occurs only in rodents, and not in any significant amount in the brains of adult primates. However, a new carbon-dating procedure shows that the adult human brain does actually continue to create new neurons.
According to an article by Spalding et al. published more recently in the journal Cell, such neuroplasticity occurs to a much greater degree than previously thought. The authors base their research on levels of carbon isotope 14 (14C) that were released into the atmosphere during aboveground nuclear bomb tests between 1945 and 1963. Dividing cells require carbon, so the 14C released into the atmosphere during the era of nuclear testing made its way into the cells of people who were alive at the time. Levels of 14C that were incorporated into the DNA of dividing cells correlate with levels of 14C in the atmosphere at the time the cells divided, and since carbon levels have declined at a predictable rate since the nuclear tests, measuring the 14C in cells can show how old they are.
Spalding et al. show that neurogenesis in humans occurs only in the hippocampus. They found evidence that a subpopulation of hippocampal neurons continually renews itself at a rate of about 700 new neurons per day, while other hippocampal cells are non-renewing. The annual turnover rate of about 1.75% is the same for men and women and declines slightly with age.
The researchers were able to determine that the renewing cells play an important role in certain types of brain function. Long-term potentiation, the process by which learning and memory can occur, depends heavily on new cells produced in the part of the hippocampus knows as the dentate gyrus.
Glia Cells Prune Over-Abundant Neurons
The brain contains neurons, which transmit electrical impulses, and glia, which protect and support neurons. New evidence suggests that some types of glia also play a role in pruning back overabundant neurons that are produced as the brain develops in utero.
Researcher Beth Stevens reports that astrocytes secrete a protein called transforming growth factor beta (TGF-beta). TGF-beta is a cytokine, or regulating protein, that activates brain microglia to initiate a complement cascade (C1 to C3), a series of chemical changes that destroy unnecessary neurons and synapses.
The various proteins involved in a complement cascade are numbered. This complement cascade starts with C1q and is continued by C4, C2, and C3, which initiate phagocytosis (or eating up) of the axon terminals of the underutilized neurons, sparing those that are active.
Inflammation and other changes in glia could cause either deficient or excess pruning of neurons, which has been thought to occur in neuropsychiatric disorders such as autism or schizophrenia.
Psychiatric Revolution: Changes in Behavior Are Associated with Dendritic Spine Shape and Number
New research shows that cocaine, defeat stress, the rapid-acting antidepressant ketamine, and learning and memory can change the size, shape, or number of spines on the dendrites of neurons. Dendrites conduct electrical impulses into the cell body from neighboring neurons.
Cocaine
Several researchers, including Peter Kalivas at the Medical University of South Carolina, have reported that cocaine increases the size of the spines on the dendrites of a certain kind of neurons (GABAergic medium spiny neurons) in the nucleus accumbens (the reward center in the brain). This occurs through a dopamine D1 selective mechanism. N-acetylcysteine, a drug that can be found in health food stores, decreases cocaine intake in animals and humans, and also normalizes the size of dendritic spines.
Depression
Depression in animals and humans is associated with decreases in Rac1, a protein in the dendritic spines on GABA neurons in the nucleus accumbens. Rac1 regulates actin and other molecules that alter the shape of the spines.
In an animal model of depression called defeat stress, rodents are stressed by repeatedly being placed in a larger animal’s territory. Their subsequent behavior mimics clinical depression. This kind of social defeat stress decreases Rac1 and causes spines to become thin and lose some function. Replacing Rac1 returns the spines to a more mature mushroom shape and reverses the depressive behavior of these socially defeated animals. Researcher Scott Russo has also found Rac1 deficits in the nucleus accumbens of depressed patients who committed suicide. Russo suggests that decreases in Rac1 are responsible for the manifestation of social avoidance and other depressive behaviors in the defeat stress animal model, and that finding ways to increase Rac1 in humans would be an important new target for antidepressant drug development.
Another animal model of depression called chronic intermittent stress (in which the animals are exposed to a series of unexpected stressors like sounds or mild shocks) also induces depression-like behavior and makes the dendritic spines thin and stubby. The drug ketamine, which can bring about antidepressant effects in humans in as short a time as 2 hours, rapidly reverses the depressive behavior in animals and converts the spines back to the larger, more mature mushroom-shape they typically have.
Learning and Extinction of Fear
Researcher Wenbiao Gan has reported that fear conditioning can change the number of dendritic spines. When animals hear a tone paired with an electrical shock, they begin to exhibit a fear response to the tone. In layer 5 of the prefrontal cortex, spines are eliminated when conditioned fear develops, and are reformed (near where the eliminated spines were) during extinction training, when animals hear the tones without receiving the shock and learn not to fear the tone. However, in the primary auditory cortex the changes are opposite: new spines are formed with learning, and spines are eliminated with extinction.
Editor’s Note: It appears that we have arrived at a new milestone in psychiatry. In the field of neurology, changes seen in the brains of patients with strokes or Alzheimer’s dementia have been considered “real” because cells were obviously lost or dead. Psychiatry, in comparison, has been considered a soft science because neuronal changes have been more difficult to see and illnesses were and still are called “mental.” Now that new technologies have made a deeper level of precision, observation, and analysis possible, we know that the brain’s 12 billion neurons and 4 times as many glial cells are exquisitely plastic–capable of biochemical and structural changes that can be reversed using appropriate therapeutic maneuvers.
The changes associated with abnormal behaviors, addictions, and even normal processes of learning and memory now have clearly been shown to correspond with the size, shape, and biochemistry of dendritic spines. These subtle, reproducible changes in the brain and body are amenable to therapeutic intervention, and are now even more demanding of sophisticated medical attention.