Mood-Stabilizing Drugs Increase Growth in Hippocampal Neurons
Lithium is known for protecting neurons by inducing neurotrophic factors and inhibiting cell death factors. In a new study, other mood-stabilizing drugs had similar neuroprotective and neurotrophic effects on cultured neurons from the hippocampus.
At the 2014 meeting of the International Society for Bipolar Disorders, CH Lee et al. presented evidence that lithium, carbamazepine, valproic acid, and lamotrigine all increase the outgrowth of dendrites from these cultured neurons. Therapeutic levels of these drugs increased the production of proteins like brain-derived neurotrophic factor (BDNF), postsynaptic density protein-95 (PSD-95), neurolignin 1 (NLG 1), beta-neurexin, and synaptophysin. However, so far only lithium has been shown to increase the volume of the human hippocampus as measured with MRI.
Genetic Test Predicts Risk of Severe Rash While Taking Carbamazepine
Carbamazepine (also known by its trade name Tegretol or, for extended release, Equetro) is one of the most widely used drugs for the treatment of epilepsy, and is relatively underutilized in the treatment of bipolar disorder. One of the reasons is fear of a rare serious rash or other side effects.
The risk of the serious rash ranges from about one in 5,000 to one in 10,000. Loss of white blood cells that fight infection (a condition called agranulocytosis) occurs in about one in 20,000 people taking carbamazepine, while a decrease in white blood cells, red blood cells, and platelets (aplastic anemia) occurs in about one in 100,000 patients.
There is no way of predicting who will develop the blood disorders in reaction to carbamazepine use. A patient should contact their doctor and get a white blood cell count if they develop some warning signs of these conditions, such as a fever or sore throat without other explanation or signs of bleeding or red spots under the skin (called petechiae) that could indicate low platelets.
Genetic Test for Risk of Rash
A genetic test is available that can help estimate the likelihood of the serious rash among certain populations. In those of Asian descent, particularly Han Chinese, Thai, Malaysian, and Indian populations, having a version of the gene HLA-B known as HLA-B*1502 is highly associated with developing the rash. (The odds ratio was 79.84 in a 2013 meta-analysis by Tangamornsuksan et al. in the journal JAMA Dermatology).
In those of northern European or Japanese descent, having a version of the gene HLA-A known as HLA-A*3101 is associated with the severe rash. (Odds ratio for developing the most severe rash was 25.93 in a study of Europeans published by McCormack et al. in the New England Journal of Medicine in 2011 and 10.8 in a study of Japanese published by Ozeki et al. in the journal Human Molecular Genetics in 2011). This HLA-A*3101 gene is present in about 2 to 5% of Europeans and 9% of Japanese.
A mild, non-serious rash with redness and itchiness occurs in about 5 to 10% of patients taking carbamazepine, and almost always goes away quickly upon stopping the drug. For patients taking carbamazepine who develop any rash, stopping the drug is the safest and most conservative thing to do. However, those who have taken the HLA test who know they do not have the risk genes and have only the benign rash might want to consider continuing to take the drug.
Benefits of Carbamazepine
There are a number of reasons why carbamazepine may be worthy of a treatment trial in patients with bipolar disorder who are not doing well on other agents. Carbamazepine works well in many patients with bipolar illness who have some of the common clinical predictors of a poor response to lithium. These include: having dysphoric (anxious, irritable) rather than euphoric mania, having an anxiety or substance disorder comorbidity, having had many prior episodes or rapid cycling (four or more episodes/year), not having distinct episodes with a period of wellness in between, having a sequential pattern of depression followed by mania followed by a well interval (D-M-I rather than M-D-I), having a schizoaffective disorder with delusions or hallucinations that persist after a manic or depressive episode has ended, and having no family history of mood disorders (especially bipolar disorder).
Some patients who do not respond to another anticonvulsant such as valproate do respond to carbamazepine. Patients with bipolar depression who have had a prior history of alcoholism may also do particularly well on carbamazepine. A benefit of the long-acting version of carbamazepine called Equetro is that it can be taken at bedtime and thus help with sleep and minimize daytime side effects.
Editor’s Note: Carbamazepine induces liver enzymes called CYP3A4 that increase the metabolism (breakdown) of carbamazepine and other drugs. Several drugs that inhibit 3A4 (such verapamil and erythromycin) prevent the breakdown of carbamazepine, causing blood levels of the drug to increase and produce side effects. If you are taking carbamazepine, tell your pharmacist so he or she can monitor any other drugs you are taking for potential interactions with carbamazepine.
Knowing about the rare skin and blood side effects of carbamazepine and some of the clinical predictors of a good response to the drug may be helpful in determining whether the potential benefits of carbamazepine outweigh the risks.
Antiepileptic Drugs for Bipolar Disorder Do Not Increase Risk of Suicidal Behavior
![]() A 30-year observational study published by Andrew Leon and colleagues in the American Journal of Psychiatry has found that anticonvulsants used in epilepsy and for bipolar depression (carbamazepine, lamotrigine, and valproate) do not increase suicidal behavior in bipolar patients.
A 30-year observational study published by Andrew Leon and colleagues in the American Journal of Psychiatry has found that anticonvulsants used in epilepsy and for bipolar depression (carbamazepine, lamotrigine, and valproate) do not increase suicidal behavior in bipolar patients.
Editor’s Note: The FDA gave a warning in 2009 that these anticonvulsants were associated with suicidal ideation. This was based on studies of a mixed group of psychiatry and neurological patients in acute placebo-controlled studies, where suicidal ideation is typically a reason for exclusion from the study. Leon et al. used more powerful longitudinal methods to compare the risk of suicidal ideation in individuals taking and not taking anticonvulsants and found no such increase in suicidal behavior.
This is like the FDA warning for antidepressants and suicide, which was based on data from placebo-controlled clinical trials in acute depression (where suicidal patients are excluded). When investigators used the same longitudinal methods as Leon et al. in the anticonvulsant study, they found that antidepressants actually reduced suicidal behavior by 30%.
The bottom line is that the use of anticonvulsants for bipolar disorder should not be discouraged based on the FDA warning about suicidal ideation in mixed neurological and psychiatric patients. In bipolar patients, anticonvulsants do not increase the risk of suicidal behaviors, i.e. suicidal acts or completed suicides.
Long-Term Treatment with Lithium, Valproate, or Carbamazepine: Lithium Best for Most Patients
 Shannon Stepan, Eric Peselow and Nunzio Pomara from Maimonides Medical Center in Brooklyn, NY, have analyzed naturalistic observations of long-term maintenance treatment of bipolar disorder with valproic acid, lithium, and carbamazepine and found that patients on lithium went much longer before experiencing an episode of mania or depression than patients taking carbamazepine or valproate.
Shannon Stepan, Eric Peselow and Nunzio Pomara from Maimonides Medical Center in Brooklyn, NY, have analyzed naturalistic observations of long-term maintenance treatment of bipolar disorder with valproic acid, lithium, and carbamazepine and found that patients on lithium went much longer before experiencing an episode of mania or depression than patients taking carbamazepine or valproate.
The team followed 225 outpatients for up to 124 months, or until they had a manic or depressive episode or dropped out of the study during a well phase. Ninety-eight patients took lithium, 78 took valproate, and 50 took carbamazepine. Fifty-two percent of the participants dropped out of the study during a well phase.
One hundred three patients (45.8%) had either a manic or depressive episode during the study. This included 36.7% of the patients taking lithium, 55% of patients taking valproate, and 50% of patients taking carbamazepine. Median time until a first episode was 45 months for the entire sample, 36 months for those patients on valproate, 42 months for those on carbamazepine, and 81 months for those on lithium. A statistical analysis known as a Cox regression model indicated that patients taking valproate had a significantly higher risk of having a manic or depressive episode than those taking lithium.
Editor’s note: These naturalistic data are highly consistent with a number of more controlled clinical studies. In particular, the BALANCE study by Geddes et al. (2010) reported that lithium was superior to valproate on most outcome measures in a two-year randomized study, and that the combination of lithium and valproate was significantly better than valproate alone. Read more
Dopamine D2 and D3 Agonist Pramipexole May Enhance Cognitive Function in Bipolar I Disorder
Anil Malhotra from the Zucker Hillside Hospital found that pramipexole (Mirapex), a dopamine D2 and D3 agonist used in the treatment of Parkinson’s disease, improved measures of processing speed and working memory in euthymic bipolar patients (whose average age was 42) when compared with placebo in an adjunctive clinical trial.
Editor’s Note: Bipolar patients in a euthymic phase have consistently been shown to have some degree of cognitive dysfunction that is typically correlated with the number of prior depressive and/or manic episodes they have experienced. This is one of the first studies to directly target this cognitive dysfunction with a pharmacotherapeutic agent.
Pramipexole may be of additional value among depressed patients, because in two small, placebo-controlled studies, one led by Carlos Zarate at the National Institute of Mental Health and one led by Joseph F. Goldberg in New York, pramipexole has been shown to exert acute antidepressant effects in bipolar patients in the depressive phase of the illness. The new data from Malhotra raise the possibility that there could be a two-for-one benefit when pramipexole is used in the depressive phase of bipolar illness—improvement in both depression and cognition.
Other approaches to improving cognition in patients with bipolar disorder
Oxcarbazepine May Be Helpful In Pediatric Mania
Oxcarbazepine (OXC; Trileptal) is a close structural relative of carbamazepine (CBZ; Tegretol; Equetro), but unlike CBZ, OXC is not an enzyme inducer, nor does it have CBZ’s risks of rare agranulocytosis or aplastic anemia.
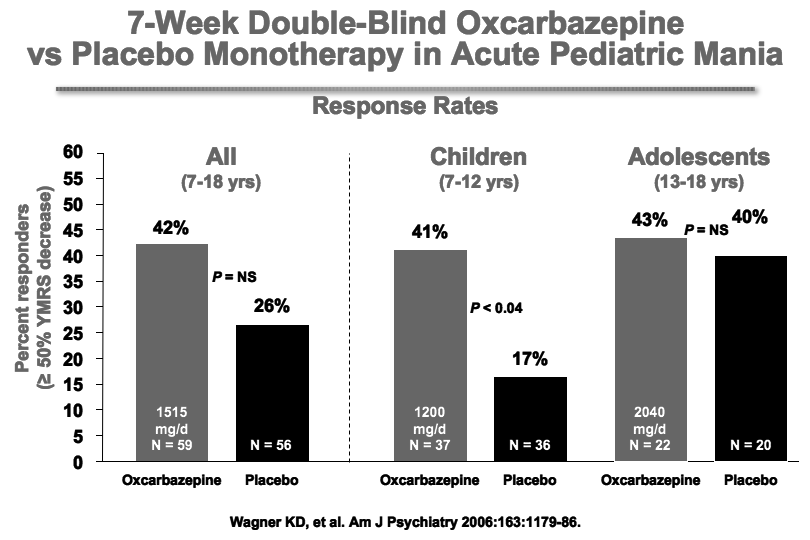
Wagner et al.’s report on OXC in the American Journal of Psychiatry in 2006 is typically cited as evidence the drug is ineffective for pediatric mania. But observe the figure:
While this was true of OXC’s efficacy in adolescents (due to a large placebo response—see rightmost column), OXC worked significantly better than placebo in children ages 7-12. These younger children often have more chronic presentations and BP-NOS. This may explain the low placebo response rate in the younger children.
Oxcarbazepine is considered helpful by many clinicians (See Post and Wozniak’s survey of expert treatment approaches to childhood illness, published in Psychiatric Annals in 2009) and should not be dismissed altogether.
Carbamazepine Extended Release Has Mixed Effects in Bipolar Children
In a poster presentation at the Pediatric Bipolar Conference in Cambridge, Massachusetts in March, Gagin Joshi from Massachusetts General Hospital (MGH) presented positive data from a study on the use of carbamazepine extended release (Equetro) in 27 children ages 6 to 12 with childhood-onset bipolar illness. These data were published this year in the Journal of Child and Adolescent Psychopharmacology.
Joshi found substantial overall improvement using an average dose of 788 mg/day, achieving blood levels averaging 6.6 mcg/l. Surprisingly, antidepressant effects were as robust as antimanic effects.
Major side effects included headache in 23% of participants, gastrointestinal upset in 18%, sedation in 15%, and dizziness in 8%. However, eleven children dropped out of the study prematurely (two for rash, three for mania, three for lack of efficacy, and three who did not participate in follow up). Joshi felt that carbamazepine extended release was a useful backup strategy, but he was not overly impressed with its overall profile in children, in part because of the high dropout rate.