Quetiapine Reduced Childhood Mania, Especially in Those with Thicker Frontal Temporal Regions
 In a symposium at the 2019 meeting of the American Academy of Child and Adolescent Psychiatry, researcher Melissa P. Delbello reported that six weeks of treatment with either lithium or quetiapine was effective in childhood mania, but quetiapine had a higher response rate of 71% versus 46% for lithium. Delbello found two types of structural changes on functional magnetic resonance imaging (fMRI). Some children had thicker frontal temporal regions, while others had thinning in these areas. The first group of patients had a 100% response to quetiapine, but only 53% of the second group responded to quetiapine.
In a symposium at the 2019 meeting of the American Academy of Child and Adolescent Psychiatry, researcher Melissa P. Delbello reported that six weeks of treatment with either lithium or quetiapine was effective in childhood mania, but quetiapine had a higher response rate of 71% versus 46% for lithium. Delbello found two types of structural changes on functional magnetic resonance imaging (fMRI). Some children had thicker frontal temporal regions, while others had thinning in these areas. The first group of patients had a 100% response to quetiapine, but only 53% of the second group responded to quetiapine.
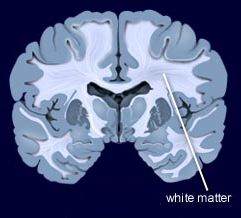
In contrast, other researchers have found lithium superior to quetiapine. Vivian Kafantaris showed that patients who respond well to lithium show improvements in white matter abnormalities. Michael Berk and colleagues found that a year on lithium was superior to quetiapine on all measures including cognition and brain imaging in patients having their first episode of mania.
Generic Seroquel XR Approved
 Earlier this year, the US Food and Drug Administration approved a generic version of Seroquel XR tablets (Quetiapine Fumarate Extended-Release Tablets), which are used to treat both depression and mania in bipolar disorder, schizophrenia, and to augment the effects of antidepressants in unipolar depression. Also known as quetiapine, the generic tablets will be available in 50 mg, 150 mg, 200 mg, 300 mg, and 400 mg doses.
Earlier this year, the US Food and Drug Administration approved a generic version of Seroquel XR tablets (Quetiapine Fumarate Extended-Release Tablets), which are used to treat both depression and mania in bipolar disorder, schizophrenia, and to augment the effects of antidepressants in unipolar depression. Also known as quetiapine, the generic tablets will be available in 50 mg, 150 mg, 200 mg, 300 mg, and 400 mg doses.
Seroquel XR is taken once per day several hours before bedtime in the acute treatment for bipolar depression (300 mg/day), mania or mixed episodes (300–600 mg/day) or their prevention (400 mg/day); or paired with antidepressants to treat unipolar depression (150–300 mg/day).
The generic tablets, which are expected to be more affordable than Seroquel XR, are produced by Pharmadax Inc.
Topiramate Added to Quetiapine Can Reduce Marijuana Craving in Young People
At the 2015 meeting of the American Academy of Child and Adolescent Psychiatry, researcher Melissa P. DelBello reported that compared to placebo, the anticonvulsant topiramate reduced marijuana craving in young people aged 12–21 who were already taking the antipsychotic quetiapine. Functional magnetic resonance imaging (fMRI) revealed that topiramate altered the activation of brain regions common to both drug craving and mood dysregulation. Topiramate could be a good treatment to reduce marijuana abuse. The antioxidant n-acetylcysteine (NAC) is another option.
Lamotrigine potentiates the antidepressant effects of quetiapine in bipolar depression
At the 2015 meeting of the International Society for Bipolar Disorders, researcher John Geddes presented an important study showing in inadequate responders to quetiapine that compared to adding placebo, adding the anticonvulsant lamotrigine to their treatment improved depression rapidly and lastingly. Some psychiatrists have been prescribing this combination to patients for some time, but this is the first formal clinical trial documenting its efficacy. The article was published online in December in the journal Lancet Psychiatry.
Researcher Guy Goodwin described details of the study, called CEQUEL, at the meeting. It included 202 patients with bipolar I or II disorder who required treatment for a depressive episode. Participants who did not respond completely to 14 days of treatment with quetiapine were prescribed either an additional dose of lamotrigine or a placebo. Lamotrigine was very slowly titrated up to maximum doses of 200mg/day. Its antidepressant effects were striking. They began early and persisted for 50 weeks. (The published article covers only the first 12 weeks.) Response rates for the combination of quetiapine and lamotrigine were 52%, compared to 22% for quetiapine alone. Remission rates were 35% for quetiapine and lamotrigine and 12% for quetiapine alone.
Folic acid interaction
Another part of the study assessed whether folic acid supplements could improve outcomes, but in fact they did the opposite, reversing the benefits of adding lamotrigine. Geddes did not have an explanation for why this might be the case. Lamotrigine can inhibit folate metabolism, and it had been thought that adding folate would be useful. Until further data are gathered on folate augmentation in patients taking the combination of lamotrigine and quetiapine, folate should be used cautiously if at all in these patients.
Possible combination with lithium
In Goodwin’s talk, he also noted lithium’s potential to lower suicide rates, premature mortality, and cognitive impairment, and to increase hippocampal and cortical volume.
Since lamotrigine was shown to potentiate the antidepressant effects of lithium in a study by Van der Loos and colleagues, and quetiapine is approved by the Food and Drug Administration for the prevention of depression as an adjunct to lithium (or valproate), there might be theoretical acute and long-term benefits to combining the three: lithium, quetiapine, and lamotrigine.
Low Dose Quetiapine Promising In Borderline Personality Disorder
Borderline personality disorder is characterized by mood instability, cognitive symptoms, impulsive or risky behavior, and disturbed interpersonal relationships. There are no Federal Drug Administration–approved treatments, but several small open studies of the atypical antipsychotic quetiapine (trade name Seroquel) have been promising. Rapid mood shifts in borderline personality disorder resemble to some extent those in bipolar disorder, for which quetiapine is an approved treatment. The drug may also curb impulsivity and self-harm. A blind, placebo-controlled study by Donald W. Black and colleagues published in the American Journal of Psychiatry in 2014 compared a low dose of quetiapine (150mg/day) with a moderate dose (300mg/day) and with placebo for the treatment of borderline personality disorder. The low dose of quetiapine led to significant improvement over the other doses, particularly reducing verbal and physical aggression.
The study included 95 participants randomized to each of the three treatment groups. All met DSM-IV criteria for borderline personality disorder, and each participant received eight weeks of active treatment. (One week of 50mg/day followed by seven weeks of 150md/day for the low dose group, and one week of 50mg/day followed by 3 weeks of 150mg/day and 4 weeks of 300mg/day for the moderate dose group.)
Eighty-eight percent of the participants experienced an adverse event during the study, including sedation, dry mouth, increased heart rate, or decrease in blood pressure. None were serious. Sedation was most common in the group receiving moderate doses of quetiapine.
All groups improved over the 8-week study, particularly during weeks 2–6. Response rates of participants who completed the study were 82% for the low dose group, 74% for the moderate dose group, and 48% for placebo. (Large benefits from placebo are common in studies of borderline personality.) Improvement in symptoms was greatest in the low dose quetiapine group, significantly higher than the moderate dose quetiapine group. Time to improvement was shorter on quetiapine than on placebo.
Combination of Lamotrigine and Quetiapine Superior to Quetiapine Alone
At a recent scientific meeting, researcher John Geddes and colleagues reported that compared to adding placebo to the treatment of bipolar depressed patients already receiving the atypical antipsychotic quetiapine, adding the mood stabilizing drug lamotrigine led to significant improvements in their illness. Lamotrigine was slowly titrated to doses of 200mg/day. (Slowly increasing dosage is important because a serious rash is a possible side effect of lamotrigine, occuring in about one in 5,000 individuals exposed.)
Researcher Charles Bowden found in 2000 that adding lamotrigine to valproate improved its effectiveness, as Marc van der Loos found in 2008 with lamotrigine and lithium. Thus it appears that adding lamotrigine to a mood stabilizer or to an atypical antipsychotic like quetiapine is a good second-line option in the treatment of bipolar depression. While lamotrigine is not FDA-approved for the acute treatment of depression, this approach is worthy of consideration, and could be of immediate clinical use. It provides an alternative to adding a unimodal antidepressant, which recent meta-analyses have indicated is not effective and which can increase switches into mania, cycle acceleration, or even treatment resistance in patients with bipolar disorder.
Lithium and Quetiapine Have Similar Efficacy in Bipolar Disorder
In a recent study comparing the efficacy of lithium and the second-generation antipsychotic quetiapine, the drugs had remarkably similar results. Researcher Andrew Nierenberg et al. presented the results at the 2014 meeting of the American Society of Clinical Psychopharmacology. In the 6-month study called CHOICE (Clinical Health Outcome Initiative Comparative Effectiveness), 482 patients received either lithium or quetiapine in addition to other medications in a manner consistent with clinical practice. For the purposes of the study, those receiving lithium could not receive quetiapine or another antipsychotic, and those receiving quetiapine could not receive lithium or another antipsychotic, but both groups could receive other types of adjunctive medications.
By the end of the 6-month study period, most patients had improved substantially, but only about a quarter of each group became truly well. The researchers suggest that patients may need a longer period of treatment or other interventions such as psychotherapy or combination treatment. Clinicians were told to use the maximum dose of lithium or quetiapine that each patient could tolerate. Mean maximum doses were 1007.5mg of lithium and 344.9mg of quetiapine.
One surprise for the researchers was that 24% of lithium patients and 27% of quetiapine patients required no other medications and improved on monotherapy.
While results were very similar for two drugs, lithium produced slightly greater side effects and produced slightly better results in patients with anxiety. This may have been due to those patients also receiving benzodiazepines, and the researchers are analyzing data to see whether the patients with anxiety did indeed receive this kind of adjunctive treatment. Quetiapine was slightly better in patients who had more manic symptoms.
In another surprise finding, patients with bipolar II disorder fared better overall than patients with bipolar I disorder. Patients with higher suicide risk did worse than those with lower suicide risk.
Long-term Treatment Response in Bipolar Illness
Willem Nolen, a researcher who has spent 40 years studying unipolar and bipolar disorder, recently retired from his position at Groningen Hospital in the Netherlands. In February, his retirement was celebrated with a symposium where he and other researchers discussed some of their important findings from the last several decades.
Nolen recently published a double-blind randomized study showing that in patients who were initially responsive to monotherapy with quetiapine (Seroquel), continuing the drug (at doses of 300-800mg/night) or switching to lithium were both more effective than switching to placebo over 72 weeks of long-term follow-up.
This study shows that quetiapine, which is only FDA-approved for long-term preventative treatment when used in combination with lithium or valproate (Depakote), also has efficacy when used as monotherapy.
Lithium is Highly Effective in Long-term Prevention
Nolen’s work also adds to an impressive amount of literature showing that lithium is highly effective in long-term prevention. This case is especially noteworthy because lithium was effective even in patients who had initially been selected for their response to quetiapine. (Studies that use this kind of “enriched sample” can only claim that quetiapine has long-term efficacy in those patients who initially respond well to the drug.) The data on lithium are even more impressive since the patients in this study were not enriched for lithium response.
Nolen has also conducted multiple studies of lithium, but optimal doses and target blood levels of the drug remain controversial. The therapeutic range of lithium is usually considered to be 0.6 to 1.2 meq/L, but some have argued that lower levels may still be effective. In a new analysis of those patients in the quetiapine study who were switched to lithium treatment, Nolen found that only lithium levels above 0.6 meq/L produced better results than placebo in long-term prophylaxis. Read more
Quetiapine May Be an Effective Monotherapy for Bipolar I
 An article published by Weisler et al. last year in the Journal of Clinical Psychiatry suggests that quetiapine may be effective as a monotherapy maintenance treatment for bipolar I disorder. It has previously been shown to work in combination with lithium or divalproex and is approved by the Federal Drug Administration for this combination treatment.
An article published by Weisler et al. last year in the Journal of Clinical Psychiatry suggests that quetiapine may be effective as a monotherapy maintenance treatment for bipolar I disorder. It has previously been shown to work in combination with lithium or divalproex and is approved by the Federal Drug Administration for this combination treatment.
Adult patients diagnosed with bipolar I disorder who were currently or recently in a mood episode received open-label quetiapine in doses of 300-800mg per day for up to 24 weeks. Patients who became stable either remained on quetiapine or were switched to lithium (at doses of 0.6-1.2 mEq/L) or placebo. This double-blind phase of the study continued for up to 104 weeks.
The study began with 2,438 patients, 1,172 of whom made it to the second phase of the trial. On the main outcome measure of time to recurrence of any mood event, both quetiapine and lithium were significantly better than placebo.
Editor’s Note: In the 50% of patients with a recent mood episode who were able to be stabilized on quetiapine monotherapy, those who remained on long-term quetiapine or those who switched to lithium were both much less likely to have subsequent relapses into either depression or mania than those who switched to placebo. Whether Astra-Zeneca, the company that produces quetiapine, will file to gain Federal Drug Administration approval of quetiapine monotherapy for long-term preventive treatment is not known.
Quetiapine (Seroquel) May Be Effective in Childhood Mania
 Gagan Joshi performed an 8-week open study of quetiapine in 30 preschool children and 19 school-age children. The mean dose was 176 mg/day on average for the preschool children, and 248 mg/day for the school age children, and both appeared highly effective in treating manic symptomatology. At the 57th Annual Meeting of the American Academy of Child and Adolescent Psychiatry (AACAP) in October 2010, Joshi reported that while the drug was generally well tolerated, it was associated with significant weight gain; the preschoolers gained 3.1 lbs on average in the 8-week period, while the school age children gained an average of 7.4 lbs.
Gagan Joshi performed an 8-week open study of quetiapine in 30 preschool children and 19 school-age children. The mean dose was 176 mg/day on average for the preschool children, and 248 mg/day for the school age children, and both appeared highly effective in treating manic symptomatology. At the 57th Annual Meeting of the American Academy of Child and Adolescent Psychiatry (AACAP) in October 2010, Joshi reported that while the drug was generally well tolerated, it was associated with significant weight gain; the preschoolers gained 3.1 lbs on average in the 8-week period, while the school age children gained an average of 7.4 lbs.