Scientific Mechanisms of Rapid-Acting Antidepressants
A pyramidal cell (Photo by Bob Jacobs, Laboratory of Quantitative Neuromorphology Department of Psychology Colorado College)
At a recent symposium, researcher Francis McMahon provided electrophysiological evidence that several different types of rapid-acting antidepressants—low-dose ketamine, scopolamine, and rapastinel (a partial agonist of the neurotransmitter NMDA)—act by decreasing the inhibitory effects of GABAergic interneurons on excitatory neurons called pyramidal cells, thus increasing synaptic firing.
Researcher Ronald Duman further dissected these effects, showing that ketamine and its active metabolite norketamine reduce the steady firing rate of GABA interneurons by blocking NMDA receptors, while the partial agonist rapastinel acts on the glutamate neurons directly, and both increase the effects of a type of glutamate receptors known as AMPA. These effects were demonstrated using a virus to selectively knock out GluN2B glutamate receptor subunits in either GABA interneurons or glutamate neurons.
Increasing AMPA activity increases synapse number and function and also increases network connectivity, which can reverse the effects of stress. Duman and colleagues further showed that when light is used to modulate pyramidal cells (a process called optogenetic stimulation) in the medial prefrontal cortex, different effects could be produced. Stimulating medial prefrontal cortex cells that contained dopamine D1 receptors, but not D2 receptors, produced rapid and sustained antidepressant effects. Conversely, inhibiting these neurons blocked the antidepressant effects of ketamine. Stimulating the terminals of these D1-containing neurons in the basolateral nucleus of the amygdala was sufficient to reproduce the antidepressant effects. These data suggest that stimulation of glutamate D1 pyramidal neurons from the medial prefrontal cortex to the basolateral nucleus of the amygdala is both necessary and sufficient to produce the antidepressant effects seen with ketamine treatment.
Researcher Hailan Hu reported that NMDA glutamate receptors drive the burst firing of lateral habenula (LHb) neurons, which make up the depressogenic or “anti-reward center” of the brain and appear to mediate anhedonic behavior (loss of interest or enjoyment) in animal models of depression. Ketamine blocks the burst firing of the LHb neurons, which disinhibits monoamine reward centers, enabling ketamine’s rapid-onset antidepressant effects. This may occur because inhibitory metabotropic glutamate receptors (mGluR-2) are activated, decreasing the release of glutamate.
MGluR-2 may also help explain the antidepressant effects of acetyl-L-carnitine supplements. L-carnitine is an amino acid that is low in the blood of depressed patients. The supplement acetyl-L-carnitine (ACL) activates the DNA promoter for mGluR-2, increasing its production and thus decreasing excess glutamate release. The acetyl group of the ACL binds to the DNA promoter for mGluR-2, thus this process seems to be epigenetic. Epigenetic mechanisms affect the structure of DNA and can be passed on to offspring even though they are not encoded in the DNA’s genetic sequence.
Rapid-Onset Antidepressant Treatments
At the International College of Neuropsychopharmacology (CINP) World Congress of Neuropsychopharmacology in 2014, several presentations and posters discussed treatments that bring about rapid-onset antidepressant effects, including ketamine, isoflurane, sleep deprivation, and scopolamine.
Ketamine’s Effects
Multiple studies, now including more than 23 according to researcher William “Biff” Bunney, continue to show the rapid-onset antidepressant efficacy of intravenous ketamine, usually at doses of 0.5 mg/kg over 40 minutes. Response rates are usually in the range of 50–70%, and effects are seen within two hours and last several days to one week. Even more remarkable are the six studies (two double-blind) reporting rapid onset of antisuicidal effects, often within 40 minutes and lasting a week or more. These have used the same doses or lower doses of 0.1 to 0.2mg/kg over a shorter time period.
Attempts to sustain the initial antidepressant effects include repeated ketamine infusions every other day up to a total of six infusions, a regimen in which typically there is no loss of effectiveness. Researcher Ronald Duman is running a trial of co-treatment with ketamine and lithium, since both drugs block the effects of GSK-3, a kinase enzyme that regulates an array of cellular functions, and in animals the two drugs show additive antidepressant effects. In addition, lithium has been shown to extend the acute antidepressant effects of one night of sleep deprivation, which are otherwise reversed by a night of recovery sleep.
Ketamine’s effects are related to the neurotransmitter glutamate, for which there are several types of receptors, including NMDA and AMPA. Ketamine causes a large burst of glutamate presumably because it blocks NMDA glutamate receptors on inhibitory interneurons that use the neurotransmitter GABA, causing glutamatergic cells to lose their inhibitory input and fire faster. While ketamine blocks the effects of this glutamate release at NMDA receptors, actions at AMPA receptors are not blocked, and AMPA activity actually increases. This increases brain-derived neurotrophic factor (BDNF), which is also required for the antidepressant effects of ketamine. Ketamine also increases the effects of mTOR, a kinase enzyme that regulates cell growth and survival, and if these are blocked with the antibiotic rapamycin, antidepressant effects do not occur.
In animal studies, ketamine increases dendritic spine growth and rapidly reverses the effects of chronic mild unpredictable stressors on the spines (restoring their mature mushroom shape and increasing their numbers), effects that occur within two hours in association with its rapid effects on behaviors that resemble human depression.
About 50–70% of treatment-resistant depressed patients respond to ketamine. However, about one-third of the population has a common genetic variation of BDNF in which one or both valine amino acids that make up the typical val-66-val allele are replaced with methionine (producing val-66-met proBDNF or met-66-met proBDNF). The methionine variations result in the BDNF being transported less easily within the cell. Patients with these poorly functioning alleles of BDNF are less likely to get good antidepressant effects from treatment with ketamine.
Ketamine in Animal Studies
Researcher Pierre Blier reviewed the effects of ketamine on the neurotransmitters serotonin, norepinephrine, and dopamine. In rodents, a swim stress test is used to measure depression-like behavior. Researchers record how quickly the rodents give up trying to get out of water and begin to float instead. Blier found that ketamine’s effects on swim stress were dependent on all three neurotransmitters. For dopamine, ketamine’s effects were dependent on increases in the number of dopamine cells firing, not on the firing rate, and for norepinephrine, ketamine’s effects were dependent on increases in burst firing patterns. Each of these effects was dependent on glutamate activity at AMPA receptors. Given these effects, Blier believes that using ketamine as an adjunct to conventional antidepressants that tend to increase these neurotransmitters may add to its clinical effectiveness.
Important Anecdotal Clinical Notes
Blier reported having given about 300 ketamine infusions to 25 patients, finding that two-thirds of these patients responded, including one-third who recovered completely, while one-third did not respond to the treatment. Patients received an average of 12 infusions, not on a set schedule, but according to when they began to lose response to the last ketamine infusion. If a patient had only a partial response, Blier gave the next ketamine treatment at a faster rate of infusion and was able to achieve a better response. These clinical observations are among the first to show that more than six ketamine infusions may be effective and well tolerated. Read more
Oral Scopolamine Promising for Depression
Intravenous scopolamine has shown promise as a rapid-acting antidepressant in studies by Carlos Zarate and colleagues at the National Institute of Mental Health (NIMH). Improvement on the drug can occur within 24 hours.
In a 6-week 2012 study, an oral preparation of scopolamine was more effective than placebo as an add-on medication to the selective serotonin reuptake intake (SSRI) antidepressant citalopram. Patients who received scopolamine and citalopram had higher rates of response and remission than those who received placebo and citalopram. The scopolamine group experienced more blurred vision and dizziness, which is to be expected from an anticholinergic drug, a drug that blocks the action of the neurotransmitter acetylcholine in the brain.
Procedures with Rapid-Onset Antidepressant Effects
 At a recent scientific meeting, Carlos Zarate of the National Institute of Mental Health (NIMH) discussed a variety of rapid-onset antidepressant manipulations. The talk focused on intravenous (IV) administration of the NMDA receptor antagonist ketamine and the anticholinergic drug scopolamine.
At a recent scientific meeting, Carlos Zarate of the National Institute of Mental Health (NIMH) discussed a variety of rapid-onset antidepressant manipulations. The talk focused on intravenous (IV) administration of the NMDA receptor antagonist ketamine and the anticholinergic drug scopolamine.
IV Ketamine
Intravenous ketamine has been administered to approximately 1000 patients at research centers at Mount Sinai Hospital in New York, Yale University, and at the NIMH in Bethesda. The results have been consistent; more than half of the patients experienced a rapid onset of antidepressant effect, usually within the first 2 hours. However, the effects of intravenous ketamine tend to disappear after 3 to 5 days.
In both unipolar and bipolar depressed patients whose illness has been highly resistant to multiple treatments, ketamine also brings about a rapid-onset decrease in suicidal ideation and intent. In some cases these positive effects have lasted a week or more. Thus intravenous ketamine could potentially be used at hospitals to treat suicidal emergencies.
Scopolamine
New data indicate that intravenous scopolamine, a selective blocker of muscarinic cholinergic receptors that is used to help ward off seasickness, brings about onset of antidepressant effects in up to one day and sometimes in a matter of hours. As with ketamine, these effects occur in both unipolar and bipolar depressed patients.
Mechanisms of Action of Ketamine
Editor’s Note: New data from animal studies may be able to explain some of the mechanisms behind the rapid onset of antidepressant effects that occurs with ketamine. Ketamine causes increases in brain-derived neurotrophic factor (BDNF), which is important for the development and health of neurons. In rodents, new synaptic proteins are created following intravenous administration of ketamine.
If animals are subjected to chronic unpredictable stress, dendritic spines (on which synapses are formed) appear to shrink. However, when ketamine is administered intravenously to these animals, not only is their behavior rapidly ameliorated, but the thin dendritic spines shift back to their more mature, mushroomed-shaped form within a few hours. (A slightly slower, less dramatic change in spines occurs with scopolamine.) A loss of dendritic volume has also been demonstrated in depressed people.
The data in animals are not only valuable for their potential application in clinical treatment and emergency situations involving suicidal ideation, but they also demonstrate a mechanism by which a single administration of intravenous ketamine or scopolamine is capable not only of reversing depressive behaviors in animals, but changing dendritic spine morphology. Given this development, it may be possible to identify other treatments that induce rapid-onset antidepressant effects using other parts of the same neurological pathways.
Other Rapid-Onset Approaches: TRH and Sleep Deprivation
Intravenous thyrotropin releasing hormone (TRH) also has rapid-onset antidepressant effects within 24 hours in depressed patients, but tolerance develops after repeated use.
Surprisingly, one night of complete sleep deprivation can also bring about dramatic onset of antidepressant effects the following day in approximately 50% of severely depressed patients.
In animal studies by Ron Duman and colleagues at Yale University, administration of brain-derived neurotrophic factor (BDNF) either into a rodent’s blood or its brain has induced rapid-onset antidepressant-like effects.
Taken together, these data indicate that there are ways to bring about rapid improvement in depression, even if conventional antidepressants usually require 2 to 4 weeks to exert their maximum antidepressant effects. Read more
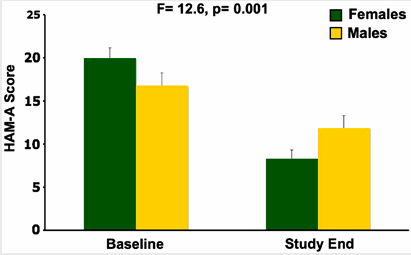
Scopolamine Produces Larger Antidepressant and Antianxiety Effects in Women Than in Men
In a study published in Neuropscyhopharmacology, three sessions of intravenous scopolamine (4µg/kg over 15 minutes) led to rapid antidepressant response in both men and women, but the magnitude of response was larger in women. Women also experienced significant reduction in anxiety, as seen below:
Editor’s Note: Scopolamine is a potent blocker of acetylcholine receptors of the muscarinic type. This can cause side effects such as dry mouth and constipation. However, when given intravenously, scopolamine produces rapid onset of antidepressant effects in both bipolar and unipolar depressed patients. This study suggests that the drug may be even more effective in women.
IV Scopolamine Brings About Rapid Onset Of Antidepressant Effects
 Frankel and colleagues from the National Institute of Mental Health presented a randomized, placebo-controlled clinical trial of intravenous (IV) scopolamine for bipolar depression at the 9th International Conference on Bipolar Disorder (ICBD) in 2011. The same group of investigators previously showed that IV scopolamine was able to induce fast-acting antidepressant responses in those with unipolar major depression. This represents a new and independent study exclusively among patients with bipolar depression.
Frankel and colleagues from the National Institute of Mental Health presented a randomized, placebo-controlled clinical trial of intravenous (IV) scopolamine for bipolar depression at the 9th International Conference on Bipolar Disorder (ICBD) in 2011. The same group of investigators previously showed that IV scopolamine was able to induce fast-acting antidepressant responses in those with unipolar major depression. This represents a new and independent study exclusively among patients with bipolar depression.
In the first phase of the study, three sessions of either IV scopolamine at 4 mcg/kg or a sham treatment were scheduled three to five days apart. In the second phase of the study, the patients were switched to the other treatment for three sessions. The results showed a rapid improvement in depression following the first session with scopolamine, more than occurred with the sham treatment. Hamilton Anxiety Rating scale (HAMA) scores also improved more on scopolamine than with the sham treatment, while Young Mania Rating Scores (YMRS) did not differ.
Editor’s note: As previously discussed in the BNN, scopolamine, an antagonist of acetylcholine muscarinic receptors, appears to exert rapid onset antidepressant effects in both unipolar and bipolar depression. When administered intravenously it takes its place with other rapidly acting antidepressant treatments, including IV ketamine, IV thyrotropin releasing hormone (TRH), and one night of sleep deprivation. The new data indicate that both unipolar and bipolar depression can respond rapidly (i.e. within a matter of hours) to certain treatments, even though most conventionally acting antidepressant modalities can take weeks to achieve maximum antidepressant effects. Read more