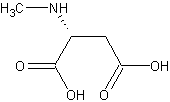
Dextromethorphan potentiates the anti-depressant effects of SSRIs
Maji et al reported in Psychiatry Research (2024) that dextromethorphan 30mg compared to placebo potentiated the antidepressant effects of SSRIs on all measures and had few side effects. It is FDA-approved to potentiate bupropion.
Now it looks like a more general property.
Scientific Mechanisms of Rapid-Acting Antidepressants
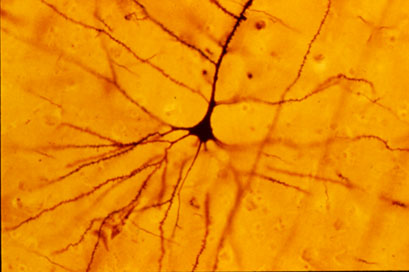
A pyramidal cell (Photo by Bob Jacobs, Laboratory of Quantitative Neuromorphology Department of Psychology Colorado College)
At a recent symposium, researcher Francis McMahon provided electrophysiological evidence that several different types of rapid-acting antidepressants—low-dose ketamine, scopolamine, and rapastinel (a partial agonist of the neurotransmitter NMDA)—act by decreasing the inhibitory effects of GABAergic interneurons on excitatory neurons called pyramidal cells, thus increasing synaptic firing.
Researcher Ronald Duman further dissected these effects, showing that ketamine and its active metabolite norketamine reduce the steady firing rate of GABA interneurons by blocking NMDA receptors, while the partial agonist rapastinel acts on the glutamate neurons directly, and both increase the effects of a type of glutamate receptors known as AMPA. These effects were demonstrated using a virus to selectively knock out GluN2B glutamate receptor subunits in either GABA interneurons or glutamate neurons.
Increasing AMPA activity increases synapse number and function and also increases network connectivity, which can reverse the effects of stress. Duman and colleagues further showed that when light is used to modulate pyramidal cells (a process called optogenetic stimulation) in the medial prefrontal cortex, different effects could be produced. Stimulating medial prefrontal cortex cells that contained dopamine D1 receptors, but not D2 receptors, produced rapid and sustained antidepressant effects. Conversely, inhibiting these neurons blocked the antidepressant effects of ketamine. Stimulating the terminals of these D1-containing neurons in the basolateral nucleus of the amygdala was sufficient to reproduce the antidepressant effects. These data suggest that stimulation of glutamate D1 pyramidal neurons from the medial prefrontal cortex to the basolateral nucleus of the amygdala is both necessary and sufficient to produce the antidepressant effects seen with ketamine treatment.
Researcher Hailan Hu reported that NMDA glutamate receptors drive the burst firing of lateral habenula (LHb) neurons, which make up the depressogenic or “anti-reward center” of the brain and appear to mediate anhedonic behavior (loss of interest or enjoyment) in animal models of depression. Ketamine blocks the burst firing of the LHb neurons, which disinhibits monoamine reward centers, enabling ketamine’s rapid-onset antidepressant effects. This may occur because inhibitory metabotropic glutamate receptors (mGluR-2) are activated, decreasing the release of glutamate.
MGluR-2 may also help explain the antidepressant effects of acetyl-L-carnitine supplements. L-carnitine is an amino acid that is low in the blood of depressed patients. The supplement acetyl-L-carnitine (ACL) activates the DNA promoter for mGluR-2, increasing its production and thus decreasing excess glutamate release. The acetyl group of the ACL binds to the DNA promoter for mGluR-2, thus this process seems to be epigenetic. Epigenetic mechanisms affect the structure of DNA and can be passed on to offspring even though they are not encoded in the DNA’s genetic sequence.
Developing Rapid Onset Antidepressant Drugs That Act at the NMDA Receptor
 For several years, researchers have been exploring potential rapid-acting treatments for unipolar and bipolar depression. Intravenous ketamine has the best-replicated results so far. A slow infusion of ketamine (0.5mg/kg over 40 minutes) produces a rapid onset of antidepressant effects in only a few hours, but the improved mood lasts only 3-5 days.
For several years, researchers have been exploring potential rapid-acting treatments for unipolar and bipolar depression. Intravenous ketamine has the best-replicated results so far. A slow infusion of ketamine (0.5mg/kg over 40 minutes) produces a rapid onset of antidepressant effects in only a few hours, but the improved mood lasts only 3-5 days.
Ketamine blocks the receptors of the main excitatory neurotransmitter in the central nervous system, glutamate. Glutamate is released from nerve endings and travels across the synapse to receptors on the next cell’s dendrites. There are multiple types of glutamate receptors at the dendrites, and ketamine blocks one called the NMDA receptor, which allows calcium ions to enter the cell.
Some downsides to ketamine are the brief duration of its effectiveness and its dissociative side effects. The search is on for other drugs that are free from these side effects and that could extend the duration of rapid-onset antidepressant effects.
At the 2012 meeting of the International Congress of Neuropsychopharmacology (CINP), Mike Quirk of the pharmaceutical company AstraZeneca reviewed data on the intricacies of the glutamate NMDA receptor blockade and discussed the potential of AZD6765, an NMDA receptor blocker he and his colleagues have been researching.
The more the NMDA receptor is blocked, the more psychomimetic it becomes, meaning it produces hallucinations and delusions. For example, phencyclidine (PCP or angel dust) is a potent NMDA receptor blocker and psychosis inducer. For antidepressant purposes, a less complete or less persistent NMDA receptor blockade is desired. Read more
Rare Encephalitis Affects NMDA Receptor
At the 57th Annual Meeting of the American Academy of Child and Adolescent Psychiatry (AACAP) in October 2010, Nadine Schwartz reported that in a rare type of encephalitis, antibodies specifically target and bind to N-methyl-D-aspartate (NMDA) receptors, the major receptors for excitatory neurotransmission in the brain. Individuals usually develop psychiatric symptoms before neurological ones, and were previously often thought to be malingering or inventing their illness. Eventually they may develop profound cognitive and motor deterioration and many may require treatment in an intensive care unit in order to provide adequate respiration. Studies show that children with this syndrome appear to respond to anti-immune therapeutic approaches including steroids, plasmaphoresis, and antimetabolites.
Editor’s Note: The recognition that auto-antibodies can attack the major receptors for excitatory neurotransmission in brain brings to light another potential mechanism that could explain neurochemical dysregulation in the neuropsychiatric disorders.