Long COVID ‘Brain Fog’ Confounds Doctors, but New Research Offers Hope
James C. Jackson, PsyD, a licensed psychologist specializing in neuropsychology and rehabilitation, at Vanderbilt University School of Medicine and author of a new book, Clearing the Fog: From Surviving to Thriving With Long COVID ? A Practical Guide, reports in Medscape July, 2023 : “There’s not a lot of imprecision in the term (brain fog) because it might mean different things to different patients,”
Jackson, who began treating [a patient] in February 2023, said that it makes more sense to call brain fog a brain impairment or an acquired brain injury (ABI) because it doesn’t occur gradually. COVID damages the brain and causes injury. For those with long COVID who were previously in the intensive care unit and may have undergone ventilation, hypoxic brain injury may result from the lack of oxygen to the brain.
An April 2022 study published in the journal Nature found strong evidence that SARS-CoV-2 infection may cause brain-related abnormalities, for example, a reduction in gray matter in certain parts of the brain, including the prefrontal cortex, hypothalamus, and amygdala.
Additionally, white matter, which is found deeper in the brain and is responsible for the exchange of information between different parts of the brain, may also be at risk of damage as a result of the virus, according to a November 2022 study published in the journal SN Comprehensive Clinical Medicine.“
Thus, new data suggest that long COVID is associated with inflammation and both reduced volume of several brain structures and decreases in white matter. These data suggest several novel approaches to therapy that require further study. One is low dose lithium which both increases gray matter volume and white matter integrity. Lithium also has some antiviral properties. This could be combined with anti-inflammatories that could be bought over the counter such as N-acetylcysteine (NAC), acetyl-L-carnitine, and celecoxib.
Note of caution. This is only an untested hypothesis and would need to be discussed with one’s physician before any of these options are considered.
Metabolic Changes in Brain of Bipolar at Autopsy
Highlights from the International Society for Bipolar Disorders Conference Posters and Presentations, Chicago, June 22-25, 2023
Graeme Preston reported on the brain of autopsied bipolar patients having increases aspartate and citrulline, while those with unipolar depression had decreases in the TCA cycle.
He saw increases in acetyl carnitine in manic bipolar patients versus bipolar depressed patients, which is of interest in relationship to the putative antidepressant effects of acetyl-L-carnitine in animal models of depression and in humans.
Dr. Post’s Recommendations For Treating Youth with Bipolar Symptoms
Our Editor-in-Chief, Dr. Robert M. Post, shares his personal recommendations for the treatment of children and adolescents with symptoms of bipolar disorder. Remember: Patients and family members must consult a physician about all information conveyed in the BNN. With the exception of lithium, none of the medications or supplements discussed above have been approved by the US Food and Drug Administration for use in children under 10. The findings reported here are in many cases preliminary and cannot be taken as recommendations based on the short summaries provided here. All treatment decisions must be made in conjunction with a patient’s treating physician, who is solely responsible for initiating any treatment discussed in the BNN or elsewhere.
In symptomatic and functionally impaired children, medication is almost always necessary. Many treating psychiatrists would start with an atypical antipsychotic, since there is clear evidence of the efficacy of such treatments. The side effects profile should be considered, as there is a considerable difference in the degree of weight gain associated with different atypical antipsychotics. The largest weight gains occur with olanzapine and clozapine, intermediate gains occur with aripiprazole and quetiapine, and the least gains occur with ziprasidone and lurasidone (and the latter has the advantage of being approved by the US Food and Drug Administration for the treatment of bipolar depression in children who are 10–17 years old). The addition of the diabetes drug metformin to decrease weight gain in people taking atypical antipsychotics is increasingly common.
The addition of an anticonvulsant medication (such as lamotrigine, carbamazepine/oxcarbazepine, or valproate) or the mood stabilizer lithium may be needed, as multiple studies indicate that combination treatment is typically needed in children (as in adults) to achieve a more complete response or remission.
Interestingly, oxcarbazepine was effective in younger but not older children with mania in a previous placebo-controlled study by Karen D. Wagner and colleagues published in the American Journal of Psychiatry in 2006.
Conversely, in a 2015 article in the journal JAACAP, researcher Robert Findling reported that in a placebo-controlled study of lamotrigine, 13–17-year-olds responded better than 10–12-year-olds.
Lithium treatment deserves consideration in children with classical presentations of bipolar disorder and those who have family members who have responded well to lithium treatment.
Lithium has the benefit of improving the white matter abnormalities seen in the brains of patients with early-onset bipolar disorder. Hafeman and colleagues reported in a 2019 article that children with bipolar disorder who were treated with lithium had better long-term results upon follow up than those treated with atypical antipsychotics or anticonvulsants.
There is much less scientific consensus about other adjunctive treatments for young people with additional bipolar symptoms and comorbidities, but this editor often uses several. Vitamin D3 is often low in children with psychiatric illness, and may improve mood and cognition.
The antioxidant N-acetylcysteine (NAC) helps depression, anxiety, and irritability, and is effective at treating habit-related behaviors such as trichotillomania (compulsive hair-pulling), obsessive-compulsive disorder (OCD), and drug use, including specifically reducing marijuana use in adolescents. A typical dose is 500–600 mg capsules, one capsule twice a day for one week, then two capsules in the morning and two in the evening thereafter.
Folate or folic acid may enhance antidepressant effects and those of lithium. In patients who have a particular low-functioning variant of a gene known as MTHFR, L-methylfolate is required instead of folate.
The widely-used supplement acetyl-L-carnitine (ALC) is poorly studied in children, but deserves consideration as a supplemental treatment for patients with histories of childhood adversity. In adults with depression, blood levels of ALC may be low, particularly in those with an early onset of bipolar symptoms and a history of childhood adversity (see a 2018 article by Carla Nasca in the journal PNAS). There is a modicum of evidence that ALC produces antidepressant effects in adults. ALC may also sensitize insulin receptors and normalize blood pressure.
There is increasing evidence of the role of inflammation in depression, mania, post-traumatic stress disorder (PTSD), and schizophrenia. Checking for abnormalities in inflammatory markers in the blood (especially Il-6 and CRP) may point the way to appropriate therapy with anti-inflammatory drugs such as minocycline (100 mg twice a day) or celecoxib (200 mg twice a day) in patients who do not respond fully to first-line medications.
Low Levels of Acetyl-L-Carnitine Associated with Insulin Resistance in Traumatized Children
 Researcher Carla Nasca and colleagues from the Rockefeller University reported at a late-2018 scientific meeting that depressed patients with a history of childhood adversity had low levels of the amino acid acetyl-L-carnitine and also exhibited insulin resistance. This is noteworthy because in a series of small studies, acetyl-L-carnitine supplements have had antidepressant effects. In laboratory animals, acetyl-L-carnitine also sensitizes insulin receptors. This suggests the possibility that the supplements could provide a two-for-one benefit in depressed patients with a history of adversity in childhood.
Researcher Carla Nasca and colleagues from the Rockefeller University reported at a late-2018 scientific meeting that depressed patients with a history of childhood adversity had low levels of the amino acid acetyl-L-carnitine and also exhibited insulin resistance. This is noteworthy because in a series of small studies, acetyl-L-carnitine supplements have had antidepressant effects. In laboratory animals, acetyl-L-carnitine also sensitizes insulin receptors. This suggests the possibility that the supplements could provide a two-for-one benefit in depressed patients with a history of adversity in childhood.
Scientific Mechanisms of Rapid-Acting Antidepressants
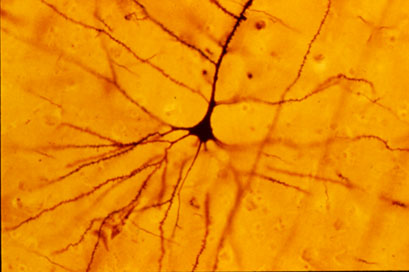
A pyramidal cell (Photo by Bob Jacobs, Laboratory of Quantitative Neuromorphology Department of Psychology Colorado College)
At a recent symposium, researcher Francis McMahon provided electrophysiological evidence that several different types of rapid-acting antidepressants—low-dose ketamine, scopolamine, and rapastinel (a partial agonist of the neurotransmitter NMDA)—act by decreasing the inhibitory effects of GABAergic interneurons on excitatory neurons called pyramidal cells, thus increasing synaptic firing.
Researcher Ronald Duman further dissected these effects, showing that ketamine and its active metabolite norketamine reduce the steady firing rate of GABA interneurons by blocking NMDA receptors, while the partial agonist rapastinel acts on the glutamate neurons directly, and both increase the effects of a type of glutamate receptors known as AMPA. These effects were demonstrated using a virus to selectively knock out GluN2B glutamate receptor subunits in either GABA interneurons or glutamate neurons.
Increasing AMPA activity increases synapse number and function and also increases network connectivity, which can reverse the effects of stress. Duman and colleagues further showed that when light is used to modulate pyramidal cells (a process called optogenetic stimulation) in the medial prefrontal cortex, different effects could be produced. Stimulating medial prefrontal cortex cells that contained dopamine D1 receptors, but not D2 receptors, produced rapid and sustained antidepressant effects. Conversely, inhibiting these neurons blocked the antidepressant effects of ketamine. Stimulating the terminals of these D1-containing neurons in the basolateral nucleus of the amygdala was sufficient to reproduce the antidepressant effects. These data suggest that stimulation of glutamate D1 pyramidal neurons from the medial prefrontal cortex to the basolateral nucleus of the amygdala is both necessary and sufficient to produce the antidepressant effects seen with ketamine treatment.
Researcher Hailan Hu reported that NMDA glutamate receptors drive the burst firing of lateral habenula (LHb) neurons, which make up the depressogenic or “anti-reward center” of the brain and appear to mediate anhedonic behavior (loss of interest or enjoyment) in animal models of depression. Ketamine blocks the burst firing of the LHb neurons, which disinhibits monoamine reward centers, enabling ketamine’s rapid-onset antidepressant effects. This may occur because inhibitory metabotropic glutamate receptors (mGluR-2) are activated, decreasing the release of glutamate.
MGluR-2 may also help explain the antidepressant effects of acetyl-L-carnitine supplements. L-carnitine is an amino acid that is low in the blood of depressed patients. The supplement acetyl-L-carnitine (ACL) activates the DNA promoter for mGluR-2, increasing its production and thus decreasing excess glutamate release. The acetyl group of the ACL binds to the DNA promoter for mGluR-2, thus this process seems to be epigenetic. Epigenetic mechanisms affect the structure of DNA and can be passed on to offspring even though they are not encoded in the DNA’s genetic sequence.
Nutritional Supplement ALC Improves Depression
A meta-analysis of 12 studies suggests that the nutrient acetyl-l-carnitine (ALC), when taken as a nutritional supplement, has antidepressant effects. The meta-analysis by researcher Nicola Veronese and colleagues appeared in the journal Psychosomatic Medicine in 2017. Veronese and colleagues found that in nine randomized controlled trials, ALC reduced depressive symptoms significantly compared to placebo. In three randomized controlled trials that compared ALC with established antidepressants, ALC showed similar effectiveness at reducing depressive symptoms while producing 79% fewer side effects. Doses of ALC ranged from 1 to 4 grams per day, and higher doses led to greater improvement.
In the comparisons with antidepressants, the other treatments included fluoxetine (Prozac), duloxetine (Cymbalta), and amisulpride (which is not approved by the US Food and Drug Administration).
Low ALC has been linked to depression. According to Veronese and colleagues, ALC deficiency can dysregulate the transport of fatty acids across the inner membrane of mitochondria. The researchers suggest several ways that ALC might contribute to an improvement in depression. One is that is seems to promote neuroplasticity in cerebral regions implicated in depression, such as the hippocampus. It could also work by increasing brain-derived neurotrophic factor (BDNF), which protects neurons and is important for learning and memory. ALC decreases release of the neurotransmitter glutamate by increasing the production of the inhibitory metabotrophic glutamate receptor (mGluR-2) on presynaptic glutamate neurons . Another way ALC might work is by normalizing lipid metabolism. Or it could modulate neurotransmitters, increasing serotonin and dopamine and protecting against stress.
In the meta-analysis, ALC produced more improvement in older patients than in younger ones. The researchers stressed the need for better treatments for older people, which may experience falls, cardiovascular disease, or increased mortality from antidepressants.
ALC also seems to improve pain syndromes, making it a good option for patients with both depression and pain symptoms.
Veronese and colleagues cited another meta-analysis that found that taking ALC in addition to an antidepressant led to lower rates of adverse events than the antidepressants alone, which helped patients adhere to their drug regimen.
Supplement Acetyl-L-Carnitine May Treat Stress and Depression
N-acetylcysteine (NAC), an antioxidant sold in health food stores, has several beneficial effects on brain and behavior. It improves depression and can reduce cravings for cocaine, alcohol, marijuana, and nicotine, and can also help control habit-driven behaviors such as gambling, compulsive hair-pulling, and symptoms of obsessive-compulsive disorder (OCD).
New research, particularly by researcher Nascaa and colleagues in 2014 and 2016, has identified a related compound, acetyl-l-carnitine (ALC), as an anti-stressor and antidepressant in animals, and researchers have begun to explore its use in people. ALC has been found to improve mitochondrial function and improve recovery from peripheral nerve damage. ALC also inhibits the release of glutamate, which can prevent depressive behaviors following stress.
A 2004 study by P. Ruggenenti and colleagues in the journal Hypertension found that in people, 1 gm of ALC taken twice daily safely improved arterial hypertension, insulin resistance, impaired glucose tolerance, and low levels of adiponectin in the blood (a risk factor for diabetes) in subjects at increased cardiovascular risk.
In a 2014 article in the Journal of Psychiatric Research, researcher S.M. Wang and colleagues reviewed evidence that ALC improves mild depression. Two randomized clinical trials indicated that ALC was more effective than placebo for mild depression. Two other randomized clinical trials showed that ALC was as effective as the antidepressants fluoxetine and amisulpride for mild depression. The supplement was as tolerable as placebo and better tolerated than fluoxetine and amisulpride. Wang and colleagues suggested that more clinical trials are needed to confirm that ALC is effective in depression.
Editor’s Note: If further clinical trials confirm the antidepressant effects of ALC, it could represent a new way to treat chronic stress and depression and regulate insulin. Together these effects could reduce the cardiovascular risks that accompany depression.
Acetly-l-carnitine May Be Effective in Treatment-Resistant Depression
 Not all patients with unipolar depression respond to the currently available antidepressants. Acetyl-l-carnitine is a compound that enhances mitochondrial function and neuroplasticity and is effective in the treatment of peripheral neuropathy (damage to the peripheral nerves, which sometimes occurs in chemotherapy or diabetes). It is now being investigated as an antidepressant for patients who have not responded to typical antidepressants.
Not all patients with unipolar depression respond to the currently available antidepressants. Acetyl-l-carnitine is a compound that enhances mitochondrial function and neuroplasticity and is effective in the treatment of peripheral neuropathy (damage to the peripheral nerves, which sometimes occurs in chemotherapy or diabetes). It is now being investigated as an antidepressant for patients who have not responded to typical antidepressants.
According to a review of the treatment by S.M. Wang et al. published in the Journal of Psychiatric Research in 2014, acetyl-l-carnitine treated depression better than placebo did in four randomized clinical studies. It was better than placebo and equally as effective as the antidepressant fluoxetine and the atypical antipsychotic amisulpride in various studies of dysthymic disorder. It also improved depressive symptoms in people with fibromyalgia and minimal hepatic encephalopathy (liver damage). The usual dose of acetyl-l-carnitine is 1 to 2 grams/day.
Editor’s Note: The role acetyl-l-carnitine will play in treating people with treatment-resistant unipolar or bipolar depression remains to be better clarified.




