Safety of Atypical Antipsychotics in Pregnancy
A 2017 article in the Journal of Clinical Psychiatry systematically reviewed data on the risks related to schizophrenia, bipolar disorder, and treatment with atypical antipsychotic medication during pregnancy. The article by researcher Sarah Tosato and colleagues suggests that a mother’s illness may be more harmful to a fetus than treatment for that illness is.
The review analyzed 49 articles about illness-related and atypical antipsychotic–related risks in bipolar disorder and schizophrenia. Tosato and colleagues found that abrupt discontinuation of treatment led to a high risk of relapse in pregnant women with bipolar disorder or schizophrenia.
Schizophrenia was linked to a slight increase in obstetric complications for mothers, while both bipolar disorder and schizophrenia were linked to a slight increase in complications for newborns. Mothers ill with schizophrenia had the highest risk for serious complications, including stillbirth, neonatal or infant death, and intellectual disability in the child.
The researchers reported that untreated bipolar disorder and schizophrenia are risk factors for birth defects, but use of atypical antipsychotics is not. Children’s neurodevelopment also does not seem to be affected by mothers’ use of atypical antipsychotics during pregnancy.
The authors suggest that, given parents agree and understand any risks involved, the least harmful choice of action is to maintain treatment of women with bipolar disorder and schizophrenia during pregnancy at the safest minimum dosage to keep their illness at bay.
Schizophrenia Drug May Treat ADHD with Impulsive Aggression
The atypical antipsychotic drug molindone was used to treat schizophrenia for decades before it was pulled from the market in 2010 for business reasons. Now Supernus Pharmaceuticals is studying whether a reformulation of the drug may be used to treat attention deficit hyperactivity disorder (ADHD) that is accompanied by impulsive aggression.
Supernus tested an extended-release form of the drug in 118 children aged 6–12 with ADHD and impulsive aggression. They received either placebo or between 12mg and 54mg per day of molindone for 39 days. Those children who received between 12mg and 36mg per day of molindone showed fewer symptoms of impulsive aggression that those who received placebo. Side effects included headache, sedation, and increased appetite. Clinical trials of molindone will continue.
Long-Acting Injectable Aripiprazole Approved for Schizophrenia
 In October, the US Food and Drug Administration (FDA) approved an injectable, long-acting version of the atypical antipsychotic aripiprazole for the treatment of adults with schizophrenia.
In October, the US Food and Drug Administration (FDA) approved an injectable, long-acting version of the atypical antipsychotic aripiprazole for the treatment of adults with schizophrenia.
The long-acting aripiprazole is administered every 4 to 6 weeks as an injection in the arm or buttocks. The company announced that it would begin releasing the drug immediately. The drug preparation for maintenance treatment is named Maintena while the preparation for acute treatment is named Aristada.
New Atypical Antipsychotic Drug Brexpiprazole Improves Depression When Added to Antidepressants
 Two studies published in the Journal of Clinical Psychiatry in 2015 suggest that the new atypical antipsychotic brexpiprazole (trade name Rexulti) safely improves depression when added to antidepressant treatment. The 6-week studies, both by Michael E. Thase and colleagues, compared brexpiprazole to placebo in people who had not responded adequately to one to three antidepressants and were taking at least one antidepressant at the time of the study.
Two studies published in the Journal of Clinical Psychiatry in 2015 suggest that the new atypical antipsychotic brexpiprazole (trade name Rexulti) safely improves depression when added to antidepressant treatment. The 6-week studies, both by Michael E. Thase and colleagues, compared brexpiprazole to placebo in people who had not responded adequately to one to three antidepressants and were taking at least one antidepressant at the time of the study.
The studies examined the effectiveness of different doses of brexpiprazole. Doses of 2mg/day and 3mg/day were more effective than placebo, while a dose of 1mg/day was not. The drug was well-tolerated by patients at each of these doses, although those taking the 3mg/day reported more side effects than those taking 2mg/day. The side effects included restless legs, weight gain, and headaches.
Like the atypical antipsychotic aripiprazole (Abilify), brexpiprazole partially blocks and partially stimulates dopamine receptors. While aripiprazole allows 61% activity at dopamine D2 receptors, brexpiprazole allows 43%. It is not yet clear how the new drug’s effects may differ from those of aripiprazole.
Another relatively new atypical antipsychotic drug, cariprazine (Vraylar) is approved by the Food and Drug Administration for schizophrenia and mania, but not yet for bipolar depression or as an add-on treatment to antidepressants in unipolar depression, although there are placebo-controlled trials showing that cariprazine can also treat these conditions.
Like aripiprazole and brexpiprazole, cariprazine also partially blocks and partially stimulates dopamine receptors. Unlike them, cariprazine is more potent at dopamine D3 receptors, which are linked to mood, motivation, and drug reward, than at D2 receptors, which are linked to motor control. It is not yet clear how these differences may change treatment outcomes or side effects.
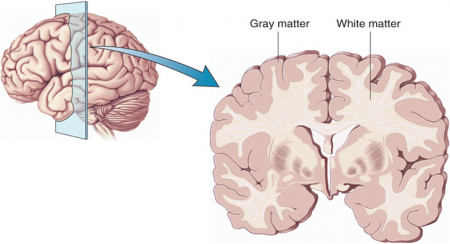
Atypical Antipsychotics May Slow Loss of Gray Matter in Schizophrenia
Progressive losses in gray matter have been observed in the cortex of people with schizophrenia, and those at high risk for the illness. In the past, studies have shown that the amount of antipsychotics a patient is exposed to is correlated with the extent of their deficits in gray matter, suggesting that antipsychotic treatment could exacerbate gray matter loss.
A new meta-analysis by Antotonio Vita and colleagues in the journal Biological Psychiatry shows that first-generation antipsychotics were associated with greater losses in gray matter compared with atypical antipsychotics, which seemed to slow the loss of gray matter.
The meta-analysis analyzed data from 18 longitudinal studies comparing a total of 1155 patients with schizophrenia to 911 healthy control participants. Magnetic resonance imaging (MRI) scans showed that over time, patients with schizophrenia lost more cortical gray matter volume. The patients’ cumulative intake of any kind of antipsychotic between MRI scans was associated with gray matter losses. But when Vita and colleagues drilled down to find differences between patients taking first-generation antipsychotics and those taking second-generation atypical antipsychotics, they found that patients with higher average daily intake of first-generation antipsychotics had greater losses in gray matter, while patients with higher average daily intake of atypical antipsychotics had less progressive losses in gray matter.
This study is the first to compare the effects of first-generation antipsychotics, which were developed in the 1960s, with those of atypical antipsychotics, which came into frequent use in the late 1980s, on cortical gray matter loss in schizophrenia. While first-generation antipsychotics are associated with the side effect of tardive dyskinesia, involuntary movements of the face and jaw, atypical antipsychotics are most commonly associated with weight gain.
Three studies have randomly assigned patients with schizophrenia to receive either first-generation or atypical antipsychotics. In these studies as well, second-generation antipsychotics were associated with smaller losses in gray matter.
The authors speculate that either second-generation antipsychotics may have neuroprotective effects, or first-generation antipsychotics may have neurotoxic effects. They also suggest that first-generation antipsychotics may not have the capacity to interfere with the natural progression of schizophrenia in terms of gray matter losses.
Future studies may investigate differences between specific antipsychotic medications’ effects on gray matter volume. Vita and colleagues reported that in the analysis, the atypical antipsychotic clozapine was associated with the least loss of gray matter of any medication in the included studies.
Editor’s Note: This study is important because it adds to findings questioning the conclusions of a large National Institute of Mental Health–sponsored study known as CATIE and a meta-analysis by John Geddes published in the journal BMJ in 2000, in which he wrote that “There is no clear evidence that atypical antipsychotics are more effective or better tolerated than conventional (first generation) antipsychotics.” Read more
Atypical Antipsychotics in Bipolar Depression
A limited number of atypical antipsychotics are approved by the Federal Drug Administration for the treatment of depression in patients with bipolar disorder. This is important to note, because the widely used traditional antidepressants that are highly effective in unipolar depression are not effective in bipolar depression. Here we review the status of the only three approved drug treatments for bipolar depression (olanzapine, quetiapine, and lurasidone) and highlight data on a promising new atypical antipsychotic, cariprazine.
Lurasidone (Latuda)
At the 2014 meeting of the International College of Psychopharmacology, researcher Joseph Calabrese reviewed the efficacy of the latest atypical antipsychotic to receive FDA approval for bipolar depression, lurasidone. In monotherapy, both low (20–60mg/day) and high doses (80–120mg/day) showed higher response rates (53% and 51%, respectively) than placebo (30%). When added to either lithium or valproate, lurasidone response (57%) again exceeded that of placebo (42%). Calabrese also indicated that all of the other secondary outcome measures were also statistically significant, including score on the Clinical Global Impressions scale for bipolar disorder, time to response, percentage of remitters, time to remit, score on the Hamilton Anxiety scale, and a patient rated depression scale (QIDS).
Lurasidone is also approved for schizophrenia at higher doses (up to 160mg/day). At least twice as much of the drug is absorbed when food is in the stomach, so it is recommended that patients take it one to two hours after dinner or after a snack of 350 calories or more. The drug has an excellent side effects profile, as it is weight- and metabolically- neutral (i.e. it does not increase blood glucose, cholesterol, or triglycerides).
Quetiapine (Seroquel)
The atypical antipsychotic quetiapine has been FDA-approved for bipolar depression for a number of years. It consistently performs better than placebo in bipolar depression, and unlike lurasidone, quetiapine is also FDA-approved for mania, as well as for long-term prevention of both manic and depressive episodes as an adjunct to either lithium or valproate. Quetiapine is also superior to placebo for prevention of both manic and depressive episodes as a monotherapy, but is not FDA-approved for this indication. A good target dose for bipolar depression is 300mg/day of the extended release preparation taken several hours prior to bed time. Higher doses of 400 to 800mg/night are used for mania and schizophrenia. Quetiapine is also FDA-approved as an adjunct to antidepressants in unipolar depression. The drug has sedative side effects, perhaps because of its potent antihistamine effects. It can also increase weight, glucose, and cholesterol slightly more than placebo.
Olanzapine and Fluoxetine
Olanzapine (Zyprexa) and a combined preparation of olanzapine and fluoxetine (Symbyax) are also approved for bipolar depression, but many guidelines suggest that these be considered secondary treatments because they are associated with weight gain and adverse metabolic effects.
Cariprazine Effective in Bipolar Depression and Mania
At the 2014 meeting of the International College of Neuropsychopharmacology, researcher Suresh Durgam presented a poster on the first study of the atypical antipsychotic cariprazine in bipolar depression. There have also been three positive placebo-controlled studies of the drug in mania. It is a dopamine D2 and D3 partial agonist, with greater potency at the D3 receptor than the atypical antipsychotic aripiprazole (Abilify). In the large placebo-controlled eight-week study, doses of 1.5mg/day were superior to placebo, but higher (3mg) and lower doses (0.75mg) were not.
Another poster presented by the same research group also reported that augmentation of antidepressants with cariprazine in unipolar depression had results that were significantly better than placebo.
Editor’s Note: While all atypical antipsychotics that have been tested for mania have antimanic efficacy (lurasidone has not been studied in mania), their antidepressant profiles differ considerably. Only the three atypical antipsychotics noted above (olanzapine/fluoxetine, quetiapine, and lurasidone) are FDA-approved for bipolar depression, and in light of recent findings, cariprazine is likely to follow soon.
The atypical antipsychotics NOT approved for bipolar depression include: aripiprazole (Abilify), risperidone (Risperidol), and ziprasidone (Geodon), with the first atypical antipsychotic clozapine and the most recent ones not yet formally tested as far as this editor is aware, including asenapine (Saphris), iloperidone (Fanapt), and paliperidone (Invega).
Only the atypical antipsychotics aripiprazole and quetiapine are FDA-approved as adjunctive treatments to antidepressants in unipolar depression, and cariprazine may soon be added to this list.
Patients with Schizophrenia Have Short Telomeres, Atypical Antipsychotics Increased Telomere Length in Animals
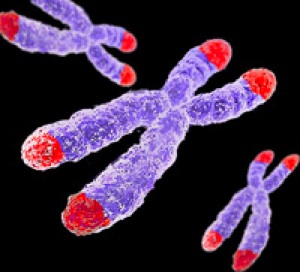 Telomeres sit at the end of DNA strands and shorten with each cell replication. A person’s percentage of short telomeres increases with aging. Telomeres also shorten with childhood adversity and as a function of number of depressive episodes.
Telomeres sit at the end of DNA strands and shorten with each cell replication. A person’s percentage of short telomeres increases with aging. Telomeres also shorten with childhood adversity and as a function of number of depressive episodes.
In a recent study, researcher Kazuya Torimi found that telomeres in white cells were shorter in 42 patients with schizophrenia compared to 56 healthy control participants.
Torimi also treated mice with various antipsychotics for 2-week periods. Treatment with atypical antipsychotics, such as risperidone, olanzapine and aripiprazole, but not typical antipsychotics like haloperidol, elongated telomere length in the hippocampus. This probably occurred through effects on serotonin.
Torimi suggests that atypical antipsychotics improve negative symptoms in part through the modulation of telomere length.
Editor’s Note: Lithium has been found to increase telomere length in patients with bipolar disorder, and appears to do this through a direct effect on telomerase, an enzyme responsible for adding to telomere length. Short telomeres are associated with a large number of medical and psychiatric illnesses.
Cariprazine for Mania Appears Safe and Well-Tolerated
At the 2014 meeting of the International College of Neuropsychopharmacology, researcher Lakshmi Latham presented a poster on three studies of the atypical atypical antipsychotic caripazine, a treatment that has not yet been approved by the Federal Drug Administration. We call it an atypical atypical because it is a partial agonist at dopamine D2 and D3 receptors, meaning it stimulates the receptors a little, but in the presence of high levels of dopamine it blocks excess activity by sitting on the receptor and preventing the actions of the excess dopamine. Aripiprazole is also a partial agonist at dopamine and serotonin 5HT1a receptors, but caripazine differs in that it has a particular affinity for the D3 receptor.
Previous analyses had revealed that cariprazine has good acute antimanic efficacy. All three studies described by Latham were randomized, double-blind, placebo-controlled three-week studies in patients with bipolar mania. In total the studies included 1065 patients, 442 of whom received placebo and 623 of whom received cariprazine.
Cariprazine doses from three studies were pooled, and ranged from 3-12 mg/day. Additional analyses evaluated the 3-6 and 9-12 mg/day groups specifically.
Approximately 70% of patients completed the study. The most common side effects included akathisia or restless legs (placebo, 5%; cariprazine, 20%), extrapyramidal disorder characterized by abnormal motor symptoms (5%, 13%), restlessness (2%, 6%) and vomiting (4%, 9%). The incidence of serious side effects was similar across the placebo and the treatment groups. Side effects that led to discontinuation of participation in the study occurred in 7% of placebo patients and 12% of cariprazine patients. Suicidal ideation was an infrequent side effect (placebo, 4; cariprazine, 2), and there were no suicide attempts.
Mean changes in weight were small (averaging 0.17kg in patients taking placebo and 0.54kg in those taking cariprazine), and the proportion of patients with 7% or higher increase in weight were similar across the two groups (both 2%). Mean changes in blood pressure and pulse were slightly greater with cariprazine and were related to dosage. Cariprazine was not associated with mean increases in electrocardiogram (EKG) parameters except for a slight increase in ventricular heart rate versus placebo (5.0 and 0.9 bpm, respectively). Mean changes in lipids and glucose were generally small and similar between groups. Levels of the hormone prolactin decreased in both groups.
Latham concluded that cariprazine treatment for three weeks was safe and well-tolerated.
More Evidence That Regular Antidepressants Do Not Work in Bipolar Depression
Psychiatrists most commonly prescribe antidepressants for bipolar depression, but mounting evidence shows that the traditional antidepressants that are effective in unipolar depression are not effective in bipolar disorder. At the 2013 meeting of the American Psychiatric Association, researcher Jessica Lynn Warner reported that among 377 patients with Bipolar I Disorder who were discharged from a hospital, those who were prescribed an antidepressant at discharge were just as likely to be remitted for a new depression than those not given an antidepressant.
The average time to readmission also did not differ across the two groups and was 205 +/- 152 days. Those patients prescribed the serotonin and norepinephrine reuptake inhibitor (SNRI) drug venlafaxine (Effexor) were three times more likely to be readmitted than those not prescribed antidepressants.
These naturalistic data (generated from observations of what doctors normally do and information in the hospital’s clinical notes) resemble those from controlled studies. In the most recent meta-analysis of antidepressants in the treatment of bipolar depression (by researchers Sidor and MacQueen), there appeared to be no benefit to adding antidepressants to ongoing treatment with a mood stabilizer over adding placebo. Randomized studies by this editor Post et al. and Vieta et al. have shown that venlafaxine is more likely to bring about switches into mania than other types of antidepressants such as bupropion or selective serotonin reuptake inhibitors (SSRIs).
In addition, a naturalistic study published by this editor Post et al. in the Journal of Clinical Psychiatry in 2012 showed that the number of times antidepressants were prescribed prior to a patient’s entrance into a treatment network (the Bipolar Collaborative Network) at an average age of 40 was related to their failure to achieve a good response or a remission for a duration of at least six months during prospective treatment.
Editor’s Note: Antidepressants are still the most widely used treatments for bipolar depression, and their popularity over more effective treatments (mood stabilizers and some atypical antipsychotics) probably contributes to the fact that patients with bipolar disorder receiving typical treatment in their communities spend three times as much time in depressions than in manic episodes. Using other treatments first before an antidepressant would appear to do more to prevent bipolar depression. These treatments include mood stabilizers (lithium, lamotrigine, carbamazepine, and valproate); the atypical antipsychotics that are FDA-approved for monotherapy in bipolar depression, lurasidone (Latuda) and quetiapine (Seroquel); and the combination of olanzapine and fluoxetine that goes by the trade name Symbiax.
Evidence from several sources suggests that the SNRI venlafaxine may be a risk factor for switches into mania and lead to re-hospitalizations. Other data suggest that in general, in bipolar depression, augmentation treated with antidepressants should be avoided in several cases: in childhood-onset bipolar depression, in mixed states, and in those with a history of rapid cycling (4 or more episodes per year).
Risperidone Trumps Valproate and Placebo for Treatment of Young Children with Mania
 At another symposium at the annual meeting of the American Academy of Child and Adolescent Psychiatry, Bob Kowatch of Ohio State University discussed a controlled trial of valproate, risperidone, and placebo in children 3 to 7 years of age (average age 5.5) with a diagnosis of bipolar I disorder and a Young Mania Rating Scale score (YMRS) greater than 20 at baseline. All of the children were severely ill with an average Clinical Global Assessment of Severity (CGAS) score of 44. Seventy-six percent had comorbid attention deficit hyperactivity disorder (ADHD) and 15% had an anxiety disorder. Valproate doses started at 10mg/kg and were increased after 4 days to achieve blood levels of 80 to 100µg/ml. The average dose of valproate was 300mg/day and the average blood level was 88 µg/ml. Risperidone was started at 0.25mg and increased as needed. The average dose of risperidone was 0.5mg per day.
At another symposium at the annual meeting of the American Academy of Child and Adolescent Psychiatry, Bob Kowatch of Ohio State University discussed a controlled trial of valproate, risperidone, and placebo in children 3 to 7 years of age (average age 5.5) with a diagnosis of bipolar I disorder and a Young Mania Rating Scale score (YMRS) greater than 20 at baseline. All of the children were severely ill with an average Clinical Global Assessment of Severity (CGAS) score of 44. Seventy-six percent had comorbid attention deficit hyperactivity disorder (ADHD) and 15% had an anxiety disorder. Valproate doses started at 10mg/kg and were increased after 4 days to achieve blood levels of 80 to 100µg/ml. The average dose of valproate was 300mg/day and the average blood level was 88 µg/ml. Risperidone was started at 0.25mg and increased as needed. The average dose of risperidone was 0.5mg per day.
On the main outcome measure of decrease in the YMRS score risperidone was substantially more effective than placebo, while valproate showed only marginal nonsignificant effects. However on the Clinical Global Impressions (CGI) scale for improvement in illness, risperidone showed 87% response, valproate 75% response, and placebo no response. In terms of 50% reduction in the YMRS score, this endpoint was achieved in 88% on risperidone, 50% valproate, and 15% on placebo.
Weight gain was mild on valproate and substantially more on risperidone. Risperidone was also associated with increases in insulin and prolactin.
The effect size (the size of the change the drug brought about in this study, which is calculated by dividing the mean difference between the experimental group and the control group by the standard deviation) for risperidone was extraordinarily large (3.58); very large for valproate (1.66), and moderate for placebo (0.56). The odds of getting well were 5 times greater than placebo for risperidone and 1.9 times greater than placebo for valproate.
Editors note: These data in very young children (aged 3 to 7) resemble other controlled data in the literature about the treatment of older children and adolescents, indicating a superiority of atypical antipsychotics over placebo and a greater magnitude of effect achieved with atypicals than with valproate. Based on these new data and the Federal Drug Administration (FDA) approval of several atypical antipsychotics for children with bipolar illness from ages 10 to 17, Dr. Kowatch recommended a new treatment algorithm for childhood onset bipolar disorder. Read more