Risperidone Trumps Valproate and Placebo for Treatment of Young Children with Mania
 At another symposium at the annual meeting of the American Academy of Child and Adolescent Psychiatry, Bob Kowatch of Ohio State University discussed a controlled trial of valproate, risperidone, and placebo in children 3 to 7 years of age (average age 5.5) with a diagnosis of bipolar I disorder and a Young Mania Rating Scale score (YMRS) greater than 20 at baseline. All of the children were severely ill with an average Clinical Global Assessment of Severity (CGAS) score of 44. Seventy-six percent had comorbid attention deficit hyperactivity disorder (ADHD) and 15% had an anxiety disorder. Valproate doses started at 10mg/kg and were increased after 4 days to achieve blood levels of 80 to 100µg/ml. The average dose of valproate was 300mg/day and the average blood level was 88 µg/ml. Risperidone was started at 0.25mg and increased as needed. The average dose of risperidone was 0.5mg per day.
At another symposium at the annual meeting of the American Academy of Child and Adolescent Psychiatry, Bob Kowatch of Ohio State University discussed a controlled trial of valproate, risperidone, and placebo in children 3 to 7 years of age (average age 5.5) with a diagnosis of bipolar I disorder and a Young Mania Rating Scale score (YMRS) greater than 20 at baseline. All of the children were severely ill with an average Clinical Global Assessment of Severity (CGAS) score of 44. Seventy-six percent had comorbid attention deficit hyperactivity disorder (ADHD) and 15% had an anxiety disorder. Valproate doses started at 10mg/kg and were increased after 4 days to achieve blood levels of 80 to 100µg/ml. The average dose of valproate was 300mg/day and the average blood level was 88 µg/ml. Risperidone was started at 0.25mg and increased as needed. The average dose of risperidone was 0.5mg per day.
On the main outcome measure of decrease in the YMRS score risperidone was substantially more effective than placebo, while valproate showed only marginal nonsignificant effects. However on the Clinical Global Impressions (CGI) scale for improvement in illness, risperidone showed 87% response, valproate 75% response, and placebo no response. In terms of 50% reduction in the YMRS score, this endpoint was achieved in 88% on risperidone, 50% valproate, and 15% on placebo.
Weight gain was mild on valproate and substantially more on risperidone. Risperidone was also associated with increases in insulin and prolactin.
The effect size (the size of the change the drug brought about in this study, which is calculated by dividing the mean difference between the experimental group and the control group by the standard deviation) for risperidone was extraordinarily large (3.58); very large for valproate (1.66), and moderate for placebo (0.56). The odds of getting well were 5 times greater than placebo for risperidone and 1.9 times greater than placebo for valproate.
Editors note: These data in very young children (aged 3 to 7) resemble other controlled data in the literature about the treatment of older children and adolescents, indicating a superiority of atypical antipsychotics over placebo and a greater magnitude of effect achieved with atypicals than with valproate. Based on these new data and the Federal Drug Administration (FDA) approval of several atypical antipsychotics for children with bipolar illness from ages 10 to 17, Dr. Kowatch recommended a new treatment algorithm for childhood onset bipolar disorder.
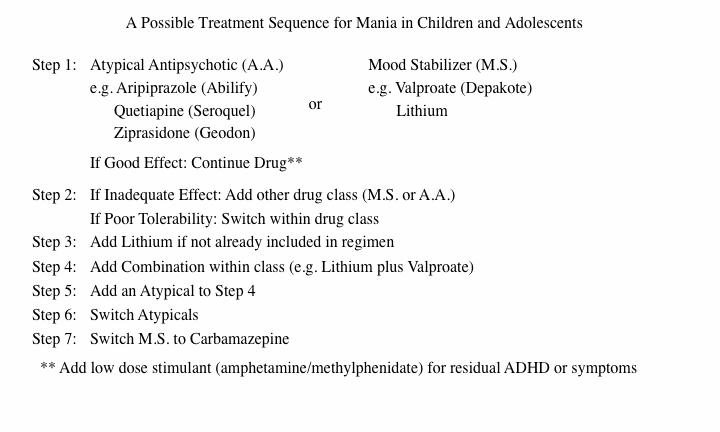
In a consensus statement on treatment guidelines for children published by Kowatch et al. in the Journal of the American Academy of Child and Adolescent Psychiatry in 2005, the authors suggested starting treatment with either an atypical antipsychotic, a mood stabilizing anticonvulsant, or lithium.
Kowatch’s Revised Treatment Algorithm
Stage I-A or B
A. In a very young child with manic illness, Kowatch now recommends starting treatment with one of the atypical antipsychotics such as quetiapine, aripiprazole, or risperidone.
B. An alternative to treatment with quetiapine, aripiprazole, or risperidone would begin with lithium or valproate. The atypical antipsychotics olanzapine and ziprasidone would also be included as part of this option because of the former’s risk of weight gain and the latter’s lack of FDA approval for children and adolescents.
In Stage II Kowatch recommends adding the type of drug not used in Stage I (lithium or an anticonvulsant added to the initial atypical, or an atypical added to initial treatment with lithium or an anticonvulsant).
In Stage III one more medication is added so that the regimen consists of two mood stabilizers and an atypical or two atypicals and a mood stabilizer.
Editors Note: This sequencing of treatments reflects the consensus of many clinicians that childhood onset bipolar disorder is difficult to treat and is associated with severe symptomatology that often dramatically impairs a patient’s social, family, and academic life. In addition to multiple medications, psychotherapy and psychoeducation are also typically required in order to achieve optimal mood stabilization.
There is some rationale for starting some severely ill children on the combination of lithium and valproate, based on a study by Findling et al. at Case Western Reserve University. They found that while almost one half of the children with bipolar who were able to be stabilized on the combination of lithium and valproate, two thirds of these children relapsed upon randomization to either lithium or valproate monotherapy. However, most re-responded when the combination of the two drugs was re-instituted. These data provide strong controlled evidence that a majority of children with bipolar disorder in the US will not respond adequately to monotherapy with lithium or valproate, and will require the combination.
Even in the TEAM study by Karen Wagner, Barbara Geller et al. we described last week, most patients required combination treatment. Not only was risperidone superior to lithium or valproate in that study, but patients also did better in the second phase of the study when risperidone was added to one of the treatments as opposed to the combination of lithium and valproate. Given the superiority of one of the atypicals (risperidone), it can be assumed that there is a class effect and that all of the atypical antipsychotics would be as good or better that lithium or valproate.
If this is the case, then the choice of an atypical with which to begin treatment or to use adjunctively would depend on its side effects profile and long-term tolerability. A highly preliminary assessment of these factors is presented in the table below.