Lithium vs Anticonvulsants Lamotrigine and Valproate on 10 year physical illness
In a study by lars Kessing in (European Neeuropsychopharmcology Volume 84, July 2024, Pages 48-56) on 169,285 patients taking either lithium or the anticonvulsants Lamotrigine (LTG) or Valproate (VPA) for at least 10 years, there was no difference in physical outcome in any physical outcome, including chronic kidney disease, except for a higher incidence of myxedema.
Other diagnoses that showed no difference included stroke, arteriosclerosis, angina pectoris, myocardial infarction, diabetes mellitus, osteoporosis, dementia, Parkinson’s disease, chronic kidney disease and cancer (including subtypes).
Editors Note: This data further supports an already robust existing literature that lithium is not more likely to cause chronic kidney disease or other physical illnesses than other anticonvulsant treatments with the exception of hypothyroidism. Patient should be made aware of these and the related long term data that lithium is no more likely to cause chronic kidney disease than other treatments and, in some cases, these other treatments cause more end-stage renal failure. The misapprehension that lithium is more toxic than other treatments has led to the vast underutilization of this treatment. Lithium is the treatment of choice for bipolar illness and should be used earlier, more often, and more persistently. When this is done illness outcomes and patient’s well being are significantly improved.
LITHIUM IS VASTLY UNDER-UTILIZED IN BIPOLAR DISORDER LEADING TO PREMATURE DEATH AND DISABILITY: WE WANT YOU TO HELP REVERSE THIS ANOMALOUS TREND
We are looking for people who have had a good course of illness with lithium included in their treatment regimen to help spread the word that lithium works extremely well and its side effects are erroneously overestimated.
We are hoping that you, as a good responder to lithium, will start a positive chain letter to fellow patients, family members, and friends suggesting that earlier and greater use of lithium would be overwhelmingly likely to improve the lives of many individuals with bipolar illness.
Why do we need you? It is because every expert in the treatment of bipolar illness of whom I am aware of has long advocated for greater and earlier use of lithium, but with little success. Lithium is widely recognized as a first line and treatment of choice for bipolar disorder, yet its use remains miniscule. In the US somewhere between only 10 to 27% of bipolar patients are given lithium. This has tragic consequences.
Treatment outcomes of the illness remain poor with vast numbers of patients experiencing pain, disability, memory loss, and loss of many years of life expectancy from suicide, cardiovascular disease, and many other psychiatric and medical disabilities. Compared to the general population, people with bipolar illness lose between 10-15 years of life expectancy. A new study by Carvalho et al (Psychother Psychosom, 2024) of more than 50,000 patients with a first episode of mania compared to more than 250,000 matched controls have a significantly higher rate of all cause mortality and a 10 fold increase of suicide. Those treated with lithium have a significantly lower rate of both all cause mortality and of suicide.
In addition, lithium has many other assets, besides the treatment of mania, of which most people are unaware and the liabilities of its side effects profile are over estimated. Some of the positive’s of lithium are listed below. Please print this ‘list of assets of lithium out and give it to everyone who might be interested. Patients with bipolar disorder should also print it out for their treating physicians, particularly if they do not as yet have lithium in their treatment regimen.
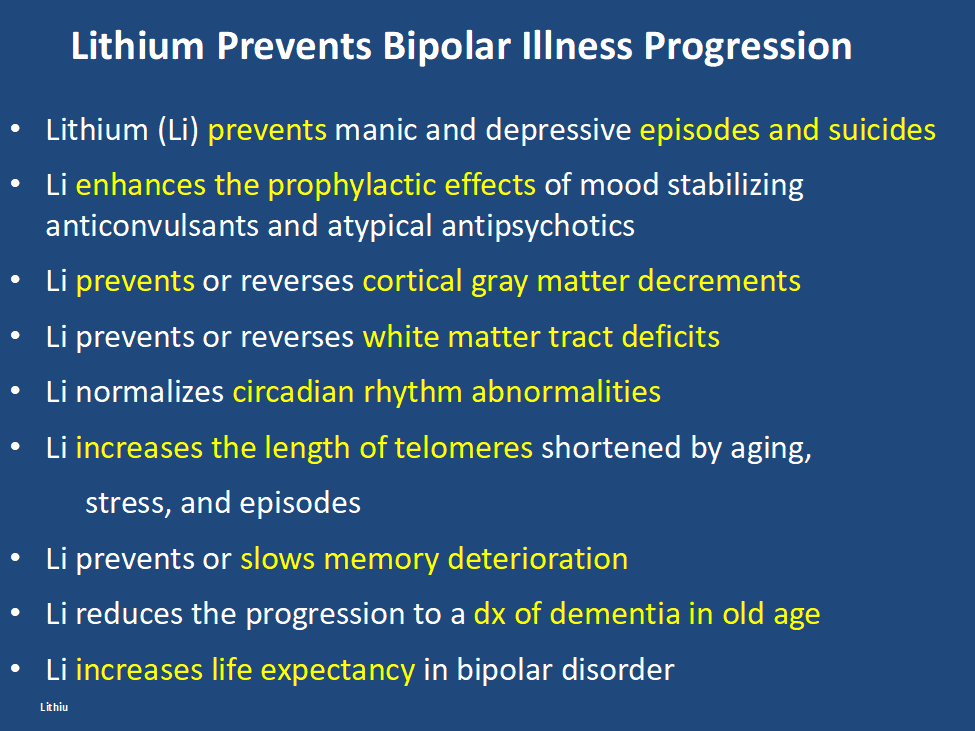
At the same time lithium’s side effects are over emphasized. The biggest concern is that lithium causes end stage kidney dysfunction eventually leading to dialysis. This is likely based on findings that individuals with bipolar disorder have an increase in most medical illnesses including chronic kidney disease compared to the general population. However, two very large trans-national studies of bipolar patients in Denmark and in Israel have found that bipolar patients treated with lithium are no more likely to get end stage renal disease than those treated with anticonvulsants such as valproate (Depakote). Lithium does cause low thyroid function in 15-25% of patients, but this is easily corrected with replacement of thyroid hormone. Many other side effects of lithium such as tremor can be managed by using lower doses.
Bottom line: Lithium gets a bad rap.
Please tell everyone you know about the new data on lithium’s relative safety and its many assets including reducing all cause mortality and suicide and restoring many years of lost life expectancy. 14 of 15 studies indicate that if lithium is started early in course of bipolar disorder it is more effective than starting it after many episodes or rapid cycling have occurred. It also works well in youngsters with bipolar disorder and better in comparison to other treatments (Hafeman et al 2020). In addition, after a first mania, patients randomized to a year of treatment with lithium do better on all outcome measures than those given a year on quetiapine (Seroquel) including manic and depressive severity, functioning, cognition, and normality of brain imaging (Berk et al 2017).
One more conceptual breakthrough: Lithium is literally the original salt of the earth. It was generated just 20 minutes after the big bang origin of the universe and is considered an essential element. Common table salt, sodium chloride, emerged only many millions of years after the big bang. Also in six studies across multiple countries, higher minute levels of lithium in the drinking water have been shown to reduce the incidence of suicide in the general population. A very low dose of lithium 150-300mg/day has also been shown to reduce the progression of mild cognitive impairment in otherwise well elderly volunteers.
Do a good thing for other people. Relay this new view of lithium to everyone you can think of in hope that they will help get the word out to many others and improve the life, functioning, and longevity of those with bipolar disorder.
Suggest and promulgate a new mantra:
“LITHIUM PREVENTS EPISODES OF BIPOLAR ILLNESS, AND PROTECTS THE BRAIN AND BODY”
Bipolar I patient show dramatic reductions in white matter integrity
Thiel et al in Neuropsychopharmacology (2024) reported that “Compared with HC [healthy controls], BD-I patients exhibited lower FA [fractional anisotropy] in widespread clusters (ptfce-FWE?< 0.001), including almost all major projection, association, and commissural fiber tracts. BD-II patients also demonstrated lower FA compared with HC, although less pronounced (ptfce-FWE?=?0.049).”
Editors Note: These data once more emphasize the importance of using lithium (Li) in bipolar disorder as it can ameliorate the deficits in white matter integrity that are so prominent in the illness. Li also improve the loss of cortical grey matter volume that evolves with illness progression. Li prevents episodes of depression and mania and reduces the incidence of suicides. That Li can reverse or ameliorate brain abnormalities in bipolar disorder is one more piece of evidence that Li should be considered a disease modifying drug (DMD) and started early in the course of illness in almost all bipolar patients. The new mantra for patients and clinicians is: Use more lithium and prevent illness progression.
Antidepressant Use and Risk of Manic Episodes in Children and Adolescents With Unipolar Depression
Suvi Virtanen, PhD; et al in JAMA Psychiatry. September 27, 2023. report a low risk of switching in youngsters with unipolar depression. However, the odds ratio for a switch were significantly elevated when there was concomitant use of anticonvulsants and antipsychotics, and there was a four fold increased risk if a parent had bipolar disorder. Thus one should be particularly careful about treating depression with antidepressants (AD) when there is a positive parental history of bipolar disorder and one should think of other options, such as lamotrigine, an atypical with good AD effects, or lithium.
More Data that Long Term Lithium Treatment Does NOT CAUSE RENAL TOXICITY (more than those on valproate).
In a recent meeting, Mark Weiser of Sheba Medical Center analysed data from “from the Clalit Health Services (CHS) database, the largest provider of health insurance in Israel, n=4.8 million, representing over 50% of the Israeli population. This study examined lithium use between the years 2000 and 2022, focusing on its impact on kidney and thyroid function…(and) compared all patients receiving lithium (n=19,433) to all patients receiving valproic acid (n=44,524). There was no different in the life-time rates of dialysis between patients treated with lithium and patients treated with valproic acid (1.03% vs 0.99%, p = 0.683). A lifetime diagnosis of hypothyroidism was more common in patients receiving lithium (21.84%) in comparison to patients treated with valproic acid (8.83%, p = <0.0001). Conclusions: In this large population study, treatment with lithium was not associated with decreased kidney function but was associated with a clinical diagnosis of hypothyroidism. These factors should be taken into account when considering treatment with lithium.”
Editors Note: In patients on lithium, overtime there are small decreases in estimated glomerular filtration rate (eGFR), but these do not differ from those seen in physiological age adjusted eGFR in the general population. These data are convergent with the large national studies of Kessing et al in Denmark and indicate that the long-held view of lithium causing undo renal toxicity are not accurate and are based on inappropriate suppositions without an adequate control group. They found more end-stage renal dysfunction in bipolar patients treated with anticonvulsants than with lithium.
THERE IS A GRAVE UNDERUTILIZATION OF LITHIUM DUE IN LARGE PART TO THE FALSE ASSUMPTION THAT IT CAUSES EXCESS RENAL TOXICITY. PATIENTS AND CLINICIANS SHOULD BE MADE AWARE OF THE NEW DATA THAT THIS IS LIKELY RELATED TO POOR METHODOLOGY AND BEGIN TO MORE FREQUENTLY THINK ABOUT USING LITHIUM — UNEQUIVOCALLY THE BEST DRUG FOR THE TREATMENT OF BIPOLAR DISORDER. LITHIUM ALSO HAS THE BEST DATA FOR REDUCING EPISODES OF BOTH DEPRESSION AND MANIA AND FOR HAVING POSITIVE EFFECTS IN PREVENTING SUICIDE. USING LITHIUM MORE OFTEN WILL UNDOUBTEDLY MARKEDLY IMPROVE PATIENTS WELL BEING AND SURVIVAL. THIS EDITOR BELIEVES THAT GIVEN LITHIUM’S MULTIFACETED ROLE IN AMELIORATING ALMOST ALL ASPECTS OF THE COURSE OF BIPOLAR DISORDER, IT SHOULD BE CONSIDERED A “DISEASE MODIFYING DRUG.” THERE ARE MULTIPLE DISEASE MODIFYING DRUGS FOR TREATMENT OF MULTIPLE SCLEROSIS, AND EXPERTS IN THAT FIELD BELIEVE THAT DISEASE MODIFYING SHOULD BE STARTED AS EARLY AS POSSIBLE AFTER FIRST DIAGNOSIS. A SIMILAR CONCLUSION WOULD NOW APPEAR APPROPRIATE FOR LITHIUM.
Of note is the other widely held reason for not using lithium more often is that it causes hypothyroidism. While this is clearly correct based on the Weiser study and many other data, patients should be aware that this well-known condition is readily correctable with thyroid hormone replacement and does not produce an undo burden on patients.
Since lithium has many other assets including: increasing hippocampal volume; protecting memory; and increasing the length of telomeres (critical to sustaining good medical and psychiatric health), its wider use in bipolar disorder should be a no brainer. However, it is likely (like most revisions in medical lore) to take 10 years or more before this re-evaluation of lithium has an impact on conventional treatment decisions, so physicians should make very active and conscious decisions about changing their routine choices of treatment for each patient with bipolar disorder.
Preliminary data on ketogenic diet
Highlights from the International Society for Bipolar Disorders Conference Posters and Presentations, Chicago, June 22-25, 2023
Georgia Ede gave a talk on the first results of 12 bipolar patients using a ketogenic diet (composed of 75% fat; 5% carbohydrates; 20% protein) as a adjunct to about 5 medications that were insufficiently effective. She saw improvement by week 3, 58% remitted, and some lost weight. She indicated that some could revert to a regular diet after the improvement achieved by children, but not in adults.
Sebari Sethi talked about 26 of 27 bipolar patients who achieved 1.3 mmole/L ketones and lost weight and showed increases in glutamate in brain measured by Glx and decreases of 11.2% in the ACC
Pediatric Bipolar Disorder is Associated with Neurocognitive Deficits
Highlights from the International Society for Bipolar Disorders Conference Posters and Presentations, Chicago, June 22-25, 2023
Maria Paula Maziero of The University of Texas Health Science Center At Houston reported that while euthymic youths with BD (bipolar disorder) exhibited significant dysfunction in working memory (WM), verbal learning, and memory domains, fluctuation between the mood states affected the type of cognitive dysfunction. They concluded: “Pediatric bipolar disorder patients have marked cognitive dysfunction involving multiple domains, especially executive measures. The severity of mood symptoms influences cognitive performance, but even euthymic persons perform lower than matched controls.“
Childhood Bullying and Maltreatment Yield A Worse Course of Bipolar Illness
Highlights from the International Society for Bipolar Disorders Conference Posters and Presentations, Chicago, June 22-25, 2023
Georgina Hosang of Bart’s & The London, Queen Mary’s School of Medicine reported that bullying and maltreatment together were associated with more suicidal behaviors than either childhood experience alone.
Lumateperone for Bipolar I or Bipolar II Depression: Few Extrapyramidal and Motor Symptoms
Highlights from the International Society for Bipolar Disorders Conference Posters and Presentations, Chicago, June 22-25, 2023
Tobie Escher of Intra-Cellular Therapies, Inc. reported on the excellent tolerability of lumateperone (42mg/day) in a “short-term population comprised 746 patients in pooled monotherapy trials (placebo, 374; lumateperone, 372) and 352 patients in the adjunctive study (adjunctive placebo, 175; adjunctive lumateperone, 177). Reported EPS [extrapyramidal symptom]-related TEAEs [treatment-emergent adverse events] were 1 patient (0.3%) with mild dyskinesia (lumateperone monotherapy), 1 (0.6%) with mild akathisia (adjunctive lumateperone), and 1 (0.3%) with severe akathisia (placebo monotherapy).”
Patterns of Pharmacotherapy for Bipolar Disorder
Highlights from the International Society for Bipolar Disorders Conference Posters and Presentations, Chicago, June 22-25, 2023
Balwinder Singh of the Mayo Clinic College of Medicine reported on “10,351 individuals from North America (n=3,985), Europe (n=3,822), and Australia (n=2,544), predominantly with cross-sectional data (80%)….They found that “Cross-sectionally, mood-stabilizing anticonvulsants (44%), second-generation antipsychotics (42%), and antidepressants (38%) were most prescribed. Lithium was prescribed in 29% of patients, primarily in Australian (31%) and European (36%) cohorts.” Lithium is remarkedly underutilized in North American cohorts.

