LITHIUM IS VASTLY UNDER-UTILIZED IN BIPOLAR DISORDER LEADING TO PREMATURE DEATH AND DISABILITY: WE WANT YOU TO HELP REVERSE THIS ANOMALOUS TREND
We are looking for people who have had a good course of illness with lithium included in their treatment regimen to help spread the word that lithium works extremely well and its side effects are erroneously overestimated.
We are hoping that you, as a good responder to lithium, will start a positive chain letter to fellow patients, family members, and friends suggesting that earlier and greater use of lithium would be overwhelmingly likely to improve the lives of many individuals with bipolar illness.
Why do we need you? It is because every expert in the treatment of bipolar illness of whom I am aware of has long advocated for greater and earlier use of lithium, but with little success. Lithium is widely recognized as a first line and treatment of choice for bipolar disorder, yet its use remains miniscule. In the US somewhere between only 10 to 27% of bipolar patients are given lithium. This has tragic consequences.
Treatment outcomes of the illness remain poor with vast numbers of patients experiencing pain, disability, memory loss, and loss of many years of life expectancy from suicide, cardiovascular disease, and many other psychiatric and medical disabilities. Compared to the general population, people with bipolar illness lose between 10-15 years of life expectancy. A new study by Carvalho et al (Psychother Psychosom, 2024) of more than 50,000 patients with a first episode of mania compared to more than 250,000 matched controls have a significantly higher rate of all cause mortality and a 10 fold increase of suicide. Those treated with lithium have a significantly lower rate of both all cause mortality and of suicide.
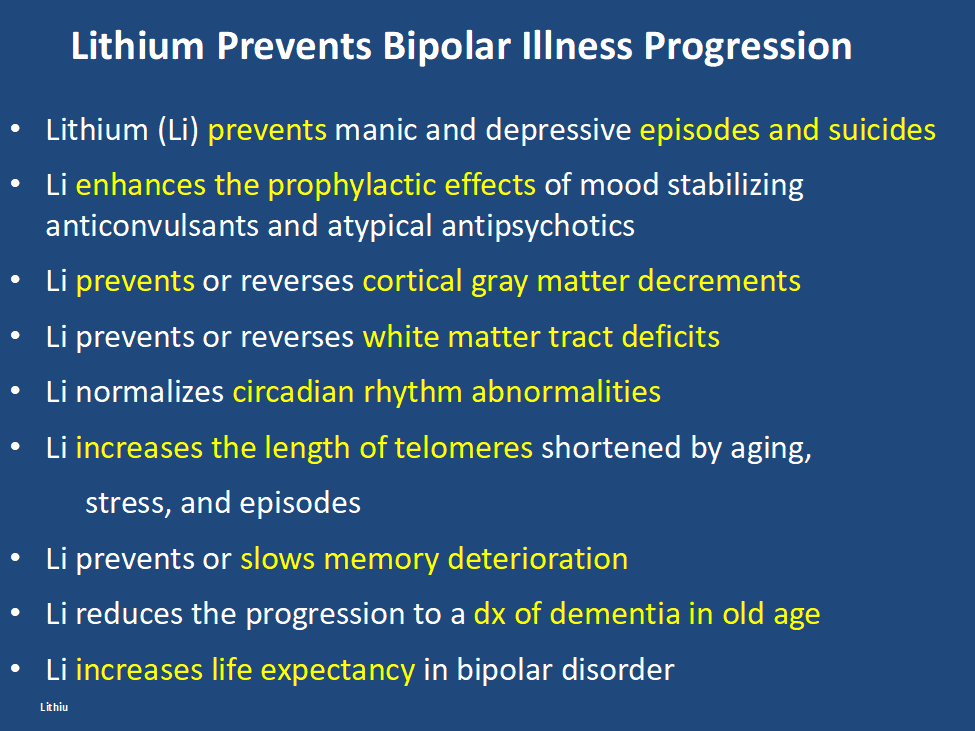
In addition, lithium has many other assets, besides the treatment of mania, of which most people are unaware and the liabilities of its side effects profile are over estimated. Some of the positive’s of lithium are listed below. Please print this ‘list of assets of lithium out and give it to everyone who might be interested. Patients with bipolar disorder should also print it out for their treating physicians, particularly if they do not as yet have lithium in their treatment regimen.
At the same time lithium’s side effects are over emphasized. The biggest concern is that lithium causes end stage kidney dysfunction eventually leading to dialysis. This is likely based on findings that individuals with bipolar disorder have an increase in most medical illnesses including chronic kidney disease compared to the general population. However, two very large trans-national studies of bipolar patients in Denmark and in Israel have found that bipolar patients treated with lithium are no more likely to get end stage renal disease than those treated with anticonvulsants such as valproate (Depakote). Lithium does cause low thyroid function in 15-25% of patients, but this is easily corrected with replacement of thyroid hormone. Many other side effects of lithium such as tremor can be managed by using lower doses.
Bottom line: Lithium gets a bad rap.
Please tell everyone you know about the new data on lithium’s relative safety and its many assets including reducing all cause mortality and suicide and restoring many years of lost life expectancy. 14 of 15 studies indicate that if lithium is started early in course of bipolar disorder it is more effective than starting it after many episodes or rapid cycling have occurred. It also works well in youngsters with bipolar disorder and better in comparison to other treatments (Hafeman et al 2020). In addition, after a first mania, patients randomized to a year of treatment with lithium do better on all outcome measures than those given a year on quetiapine (Seroquel) including manic and depressive severity, functioning, cognition, and normality of brain imaging (Berk et al 2017).
One more conceptual breakthrough: Lithium is literally the original salt of the earth. It was generated just 20 minutes after the big bang origin of the universe and is considered an essential element. Common table salt, sodium chloride, emerged only many millions of years after the big bang. Also in six studies across multiple countries, higher minute levels of lithium in the drinking water have been shown to reduce the incidence of suicide in the general population. A very low dose of lithium 150-300mg/day has also been shown to reduce the progression of mild cognitive impairment in otherwise well elderly volunteers.
Do a good thing for other people. Relay this new view of lithium to everyone you can think of in hope that they will help get the word out to many others and improve the life, functioning, and longevity of those with bipolar disorder.
Suggest and promulgate a new mantra:
“LITHIUM PREVENTS EPISODES OF BIPOLAR ILLNESS, AND PROTECTS THE BRAIN AND BODY”
GLP-1s Might Decrease the Incidence of Depression and Anxiety
Lisa O’Mary of WebMD wrote: “People taking a popular type of drug for weight loss or to manage diabetes have a lower likelihood of being newly diagnosed with depression or anxiety, according to an analysis of millions of people’s health records.
The findings were published this week by researchers from the electronic health record company Epic. Researchers looked for new diagnoses of depression or anxiety among people who started taking drugs from a class called GLP-1 agonists that can help manage blood sugar or treat obesity by mimicking hormone levels in the body that can affect appetite and blood sugar. Many people who take the drugs experience significant weight loss.
The researchers found that people with diabetes who started taking most versions of GLP-1 agonists were between 11% and 65% less likely to be newly diagnosed with depression than people with diabetes who didn’t take one of the drugs. The greatest reduction in likelihood of a new depression diagnosis was observed among people taking tirzepatide, which is sold under the brand names Mounjaro and Zepbound.”
Antidepressant Use and Risk of Manic Episodes in Children and Adolescents With Unipolar Depression
Suvi Virtanen, PhD; et al in JAMA Psychiatry. September 27, 2023. report a low risk of switching in youngsters with unipolar depression. However, the odds ratio for a switch were significantly elevated when there was concomitant use of anticonvulsants and antipsychotics, and there was a four fold increased risk if a parent had bipolar disorder. Thus one should be particularly careful about treating depression with antidepressants (AD) when there is a positive parental history of bipolar disorder and one should think of other options, such as lamotrigine, an atypical with good AD effects, or lithium.
NEW DATA ON EXTENDING THE EFFFECTS OF IV KETAMINE: Implications for countering the effects of stigma.
John Krystal of Yale U. in an article in Proceedings of the National Academy of Sci. (2023) gave new information about the anatomical and physiological effects of ketamine and about how to extend its effects. Ketamine in animals acutely (in a matter of hours) increases spines, synapses, and dendrites and physiological connectivity in neurons in the prefrontal cortex. Data now support these findings in humans with depression, but AD effects of ketamine tend to dissipate over a period of 3 to 5 days and require repeated infusions to maintain the improvement. Synaptic density can be measured with SV2A and this can be enhanced with a Navitor Pharmaceuticals drug acting on mTORC1. Surprisingly low doses of rapamycin, an inhibitor of mTOR1, extends the duration of AD effects of ketamine, increasing the response rate at 2 weeks of 13% to 41%. Ketamine restores only the spines that have been reduced in depression and rapamycin is thought to extend the duration of the restored spines. It may do this by increasing the neurotropic effects of microglia. The tripartite synapse of pre and post synaptic neurons and astrocytes, may now be better described as the tetrapartite synapse with the inclusion of microglial.
Adding psychotherapy to the effects of ketamine in PTSD increases and extends efficacy.
AD effects of ketamine can be extended with cognitive behavioral therapy. Thus, ketamine increases synaptic efficacy, synaptic density, glutamate homeostasis, and experience-dependent neuroplasticity. Extending the persistence of these effects by various chemical and psychotherapeutic mechanisms may give new ways of enhancing and prolonging the therapeutic effects of this rapidly acting agent.
Interestingly, Kaye at Yale U. reports that in contrast to transient effects of ketamine, the AD effects of therapy-assisted psilocybin appear to be very long lasting and the associated increases in spines persist for longer than 37 days. Another psychedelic MDMA that is effective in PTSD causes large increases in spines, although they are less persistent and are gone by 34 days.
Editors Note: As we have previously highlighted in the BNN, these data on ketamine and other psychedelics reversing the anatomical and physiological deficits in prefrontal neurons of depressed animals and humans (spines, dendrites, synapses and intercellular communication), give a new view of the real, reliable, and replicable neurological defects accompanying depression. The rapid correction of these neural abnormalities in conjunction with the rapid induction of antidepressant effects are not only paradigm shifting from a treatment perspective, but have major implications for the assertion that there is a neurobiological basis of depression and its treatment.
As such, these data should do much to counter the continuing stigma too often accompanying the term “mental” illness that it is somehow not as real as other medical and neurological conditions, and as it is often asserted that “it is just all in one’s head.” This picks up on the unfortunate associations and definitions of “mental” as imaginary, all in the mind, and evanescent and that mental illness can readily be countered with effort and will power such as “pulling oneself up by the bootstraps,” (the latter of which is in itself a logical impossibility.)A Case Report: Good Effect of Psilocybin Even with No Psychedelic Effects
Highlights from the International Society for Bipolar Disorders Conference Posters and Presentations, Chicago, June 22-25, 2023
Marisa Leon-Carlyle reported on a 43 yr. old man with chronic treatment refractory depression who was given psilocybin 25mg (a 5HT2A agonist) at 8:00 AM after receiving trazodone 200mg HS (which should be enough to block 80% of 5HT2A receptors). He had an excellent antidepressant response, was able to feel emotion and new love for his wife, and finally felt that doing one’s best is good enough. Obviously further systematic study is needed.
Antidepressant Effects of Psilocybin in Unipolar Depression
Highlights from the International Society for Bipolar Disorders Conference Posters and Presentations, Chicago, June 22-25, 2023
Alan Young reported on 233 patients with treatment refractory depression who were given 10mg then 25mg of psilocybin, 1 week apart.
There was intensive therapeutic support before, during, and after the ingestions. Patients experienced it as a waking dream. Response was rapid in onset and 20% response persisted at least through week 12.
Metabolic Changes in Brain of Bipolar at Autopsy
Highlights from the International Society for Bipolar Disorders Conference Posters and Presentations, Chicago, June 22-25, 2023
Graeme Preston reported on the brain of autopsied bipolar patients having increases aspartate and citrulline, while those with unipolar depression had decreases in the TCA cycle.
He saw increases in acetyl carnitine in manic bipolar patients versus bipolar depressed patients, which is of interest in relationship to the putative antidepressant effects of acetyl-L-carnitine in animal models of depression and in humans.
Positive Effects of Low-Dose Lithium (LDL)
Highlights from the International Society for Bipolar Disorders Conference Posters and Presentations, Chicago, June 22-25, 2023
Rebecca Strawbridge of the Institute of Psychiatry, Psychology & Neuroscience, King’s College London reported on 18 articles that were examined and grouped according to outcome domain (cognition, depression, mania, and related constructs e.g., suicidality). Significant benefits (versus placebo) were identified for attenuating cognitive decline, and potentially as an adjunctive therapy for people with depression/mania. Across studies, LDL (~serum level ?0.6 mmol/L) was reported to be safe.
Greater Severity of Depression in Youth With Bipolar Disorder versus Unipolar Depression
Highlights from the International Society for Bipolar Disorders Conference Posters and Presentations, Chicago, June 22-25, 2023
Aaron Silverman of the University of Toronto, CAMH found that “youth (age 13-21) with [Bipolar Disorders] compared to those with [unipolar] depression had significantly higher (more severe) ratings on depressed mood (p = .001), irritability (p = .037), anhedonia (p = .004), negative self-image (p < .001), hopelessness (p = .04), fatigue (p = .001), hypersomnia (p = .001), suicidal ideation (p = .04), and recurrent thoughts of death (p < .001).”
LITHIUM’S AMAZING DIVERSITY OF ASSETS
Editor’s Note: Lithium is vastly underutilized. There is wide spread ignorance about its many assets and misconceptions about its few side effects. Here is an update that should be of interest to potential users, family members, and clinicians.
Lithium:
- Prevents unipolar and bipolar depression
- Augments effects of antidepressants in unipolar depression
- Potentiates the effects of atypical antipsychotics in treating mania and depression
- Reduces inflammation
- Normalizes some aspects of cardiovascular risk
- Normalizes secretions for monocytes and leukocytes
- Increases neurogenesis, BCl-2, and hippocampal and thalamic volumes
- The increases in neuroprotective factors occurs at brain levels below typical therapeutic dosages
- Protects against memory deterioration
- Lowers dementia risk in old age
- Reduces suicide clinically and at minute concentrations in the water supply
- Lengthens telomeres and increases longevity
- Reduces size of lesions in models of stroke, AIDS, and Huntington’s chorea
- Normalizes circadian rhythms
- Reduces manic-like behavior induced by clock gene mutations
- Prevents calcium currents and increased firing rate in stem cells from bipolar patients
- Induces minimal to no weight gain on long term follow up
- Does not increase risk of kidney failure when given at blood levels of .6 to .8 blood levels
- Protects against spine and hip osteoporosis
Conclusion: With so many assets and so few liabilities, physicians and patients should reconsider the benefits of lithium and use it more often, not only in the few who respond to it as a monotherapy, but as a adjunct to the many other treatments of bipolar disorder. This should be a “no brainer” as lithium will very likely help some have fewer problems from their illness and may even help them live longer.
Many of these points are summarized in the open access publication: Robert M Post, The New News About Lithium: An Underutilized Treatment in The United States, Neuropsychopharmacology accepted article preview 4 October 2017; several new updates have been added from the International Society on Bipolar Disorders meeting, Chicago, June, 2023.

