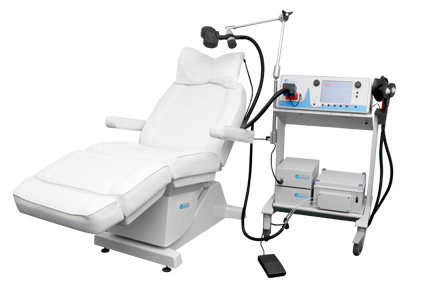
New TMS System Approved for Depression
In August the US Food and Drug Administration (FDA) approved the marketing of the MagVita TMS Therapy system from the company MagVenture. This machine can be used to provide transcranial magnetic stimulation (TMS) to patients with major depression that has not responded to antidepressant drugs. A TMS system uses magnets placed close to the head to stimulate the brain.
There are several existing systems that can provide TMS. The Neuronetics Neurostar TMS machine was the first one to be approved, in 2008. Then came Brainway’s Deep TMS machine. Now MagVenture says that the benefits of their new system include a simple design, low operating costs, no disposable components, and safety and efficacy rates comparable to those of other FDA-approved TMS devices.
Treatment with the MagVita system is typically provided five times per week for a duration of six weeks.
As TMS treatment becomes available to more patients, coverage by insurance companies is also increasing, but is still not guaranteed for patients in the US.