The N-acetylcysteine Story: A New Potential Therapy for Bipolar Illness and Substance Abuse
N-acetylcysteine (NAC), a readily available substance from health food stores, is able to reestablish glutamate homeostasis (regulation and balance) in the reward area of brain (the nucleus accumbens), reported Peter Kalivas of the University of South Carolina at the “Staging neuropsychiatric disorders: Implications for idiopathogenesis and treatment” meeting in Mojacar, Spain this past November. Kalivas reported that NAC appears to be effective across a spectrum of addictions, including cocaine, heroin, alcohol, cigarette smoking, and gambling.
Even more remarkably, NAC also appears to have positive effects in placebo-controlled studies in the treatment of patients with bipolar illness, report Mike Berk and colleagues, who are studying the same substance in Australia. Compared with placebo, patients taking adjunctive NAC showed improvement in all outcome measures, especially depression, after 3 and 6 months. In another article, also published in Biological Psychiatry in 2008, Berk’s research group demonstrated that NAC improved some negative symptoms of schizophrenia. NAC has also shown positive effects in trichotillomania and on nail-biting, suggesting that it has a variety of potential clinical uses in conditions associated with pathological compulsive behavioral patterns.
EDITOR’S NOTE: This next section is fairly technical and can be skipped over with the bottom line message that NAC dampens an overactive glutamate response in the reward area of the brain. However, some of the details about how Kalivas made these therapeutic discoveries based on data in the laboratory may be interesting to some.
N-acetylcysteine reduces cocaine and heroin re-instatement in animals and craving in humans
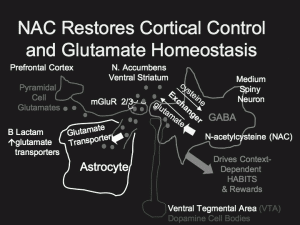
How Kalivas made his conclusions is an interesting story in and of itself. Much previous work had established that the n. accumbens was essentially a reward area of the brain and was also involved in the modulation of mood, motor activity, and motivation. The medium spiny neurons in this area of the brain have GABA as their neurotransmitter and receive both dopamine input from the midbrain ventral/tegmental area (VTA) below, as well as glutamate-mediated input from pyramidal cells in the cerebral cortex above (see schematic figure).
Increased dopamine release in the n. accumbens is associated with the reward properties of a variety of substances of abuse, and substance abuse behaviors appear to be exacerbated by inadequate prefrontal inhibition. Kalivas and associates stimulated the prelimbic part of the prefrontal cortex, which has direct synapses on the core of the n. accumbens, with either brief bursts of high-frequency stimulation or 15 minutes of low-frequency stimulation. The brief bursts of high-frequency stimulation were associated with synaptic enhancement, i.e. long-term potentiation (LTP), and the 15 minute increments of low-frequency stimulation were associated with synaptic decrements in the form of long-term depression (LTD). Kalivas studied the modulatory effects of dopamine and glutamate in this reward area of brain and found that they were highly dysregulated after chronic cocaine administration in that both LTP and LTD were weak to nonexistent.
Kalivas also found that in rats, following self-administration of i.v. cocaine by pressing a bar that delivered the cocaine reward, the animals could go through an extinction phase where they learned that pressing the bar would no longer result in a cocaine reward. However, as in the clinical situation in which cocaine addicts rapidly relapse on occasions when stress occurs, cocaine cues are present, or cocaine is readily available, the rats behaved similarly. When a cue indicated that cocaine was again available or when the animals were stressed, they would again press the lever up to 100 times per minute, even though it no longer delivered cocaine.
This cocaine reinstatement behavior was associated with huge increases in glutamate levels measured in the n. accumbens. Kalivas reasoned that if these large increases in glutamate could be suppressed, maybe reinstatement behavior would be suppressed as well. He found a way to decrease the excessive glutamate secretion via the n-acetylcysteine pathway. N-acetylcysteine is a glutathione precursor that increases the drive of a cystine-glutamate exchanger that is located outside the range of usual glutamate synapses. This in turn increases extracellular levels of glutamate and results in the increased occupation and subsequent downregulation of a different type of glutamate receptor (mGluR2/3) that is associated with inhibition of presynaptic glutamate release. This normalizes the abnormally high amount of glutamate released by stressors, conditioned cues, or cocaine.
This modulation of glutamate release onto the medium spiny neurons of the n. accumbens was in fact found to inhibit cocaine reinstatement in the animal studies. Kalivas and co-workers quickly took these preclinical observations into the clinic and found that n-acetylcysteine decreased cocaine craving in humans as well.
In further animal studies, the same large increase in glutamate release in the reward area of brain was found to occur following heroin self-administration and extinction training; heroin re-instatement behavior too was suppressed by n-acetylcysteine. Likewise, clinical placebo-controlled studies suggest that NAC decreases heroin craving in people.
Part of the reason that such high glutamate levels occurred in the n. accumbens was that prior substance abuse had downregulated glutamate transporters on glial cells (astrocytes), which normally help to remove glutamate from the synapse. NAC helped reestablish the appropriate number of glutamate transporters, which in turn helped clear synaptic glutamate.
Kalivas also found that a unique type of antibiotic (with a beta lactam structure), which currently can only be administered intravenously in humans, is associated with stimulation of the DNA promoter for the formation of more glutamate transporters in astrocytes, and thusly the antibiotic increases the rate at which excess glutamate is removed from the synapse. His research group determined that the administration of this antibiotic also blocked cocaine-induced reinstatement behavior in rats. Currently, attempts are in progress to find a more clinically feasible beta lactam antibiotic that can be given orally, in order to see whether it, as well as NAC, would help patients with a variety of addictions.
N-acetylcysteine may be useful as an adjunctive treatment for bipolar disorder
Unbeknownst to each other, while Kalivas studied NAC in the U.S., Mike Berk and colleagues in Australia completed parallel studies. Berk’s group reasoned that NAC was a glutathione precursor and thus might have antioxidant and neuroprotective effects in bipolar disorder, since increasing data have indicated an excess of inflammatory cytokines and oxidative stress in the illness, as noted on page 7. Berk and colleagues administered NAC in 500mg capsules twice per day for one week and then two capsules twice a day thereafter to patients who maintained their prior drug regimens. Berk’s group found that compared to the addition of placebo, with the addition of NAC, residual symptoms of bipolar illness that had been inadequately treated by the prior drug regimens had improved remarkably after a period of three months and continued to improve through the six-month study period.
In another double-blind study, Berk’s research group found that some of the negative symptoms in schizophrenic patients were also improved with the administration of NAC.
In 2009, another team of researchers, Grant and colleagues, published an article in the American Journal of Psychiatry reporting that NAC given to patients with trichotillomania (chronic, persistent, obsessive pulling out of one’s hair) was associated with marked improvement in this behavior after two months, compared with only a very small percentage of patients improving on placebo. What was remarkable about this trial was that the side effects were nonsignificantly different on NAC and placebo, and actually were present in a few patients on placebo and not on NAC, indicating that it was a readily tolerable drug at these doses.
NAC had also been used previously in a preparation called Mucomyst and was given to those with Tylenol overdoses because of its ability to protect against liver damage and its antioxidant properties.
NAC may work in bipolar illness and substance craving by re-regulating an overactive habit memory system
How does NAC have such a remarkably broad spectrum of therapeutic effects across so many addictions as well as in schizophrenia, bipolar illness, and trichotillomania? Consider the two kinds of memory. One is conscious or representational memory, which is dependent on circuits in the medial temporal lobe (i.e. the amygdala and hippocampus). This kind of memory allows you to remember things that happened in the past. Another form of memory, habit memory, is developed through multiple repetitions of the basic learning or conditioning paradigm, and is dependent on a basal ganglion structure called the striatum. This is the kind of memory that allows you to learn to ride a bicycle—it comes naturally without thinking, and you never forget how to do it. While habit memory is most closely linked to alterations in the dorsal part of the striatum, habit memory may also involve the motivation, reward, and motor activity-related processes mediated by the ventral striatum, also known as the n. accumbens.
Learned, context-dependent, and automatic habits may depend on this n. accumbens substrate, and this relationship may be the basis for the common therapeutic effects of NAC in widely diverse compulsive behaviors. All of these behaviors appear to represent habits that are extremely hard to suppress and are based on compulsive impulses that have some reward value, whether they involve substance abuse, nicotine, alcohol craving, gambling addiction, or hair-pulling.
It is not clear which actions of NAC improved symptoms in bipolar illness, but this editor has for many years suggested that repeated episodes of mania and depression occur increasingly autonomously, that is, they do not require precipitation by psychosocial stresses in order to emerge. One hundred years ago, Emil Kraepelin observed that initial episodes of the illness were typically associated with psychosocial stresses, but with sufficient numbers of repetitions, these occurred more independently as well. We have reasoned that this repeated occurrence of affective episodes may shift some of the neural substrates of stress and affective episodes from the conscious representational memory system in the amygdala and hippocampus into the unconscious habit system in the striatum. This would theoretically be the reason that after many repetitions, affective episodes can appear to come “out of the blue,” and why conscious efforts coded in the representational memory circuit are often unable to ward off an impending episode (even if one understands the dynamics of one’s mood). Should this analysis prove correct, NAC might be able to rebalance the circuitry of the n. accumbens to target the automaticity of affective illness recurrence, just as it is helpful in blocking the automatic habits associated with substance use or hair-pulling.
Even if this analysis proves to be incorrect, and NAC’s mechanisms of action in affective illness and schizophrenia are dissociated from those in addictions and habits such as trichotillomania, the findings are nonetheless of very considerable clinical interest from several perspectives. 1) NAC may rebalance glutamate homeostasis in the reward area of brain; 2) a compound readily found in nature can achieve this effect; and, 3) it can apparently do so with remarkable safety. NAC takes two months to work in trichitillomania and three months in bipolar illness, but may work more rapidly in the substance abuse addictions.
Potential implications for clinical treatment
It is clear that the NAC story is evolving rapidly, and currently looks almost too good to be true. We must wait for further research and clarification. In the meantime, it may be worthwhile for some patients to talk with their physicians about the potential utility of a clinical trial in their illness. Unipolar depression, bipolar disorder, and schizophrenia are too often compounded by substance abuse problems. It is hoped that NAC could ultimately provide a two-for-one benefit, i.e. helping in both the spheres of bipolar and schizophrenic affective dysregulation and substance addiction.
WARNING: Even though N-acetylcysteine (NAC) is available in health food stores, one should not begin taking the substance without first carefully discussing it with one’s treating physician. While it looks safe now, large-scale trials have not yet been reported, interactions with other drugs remain to be studied, and the possibility of idiosyncratic negative reactions on NAC or during its withdrawal are possible. If you are a patient you can assist your doctor and improve your own evaluation of any therapeutic maneuver you are trying by systematically charting your prior and future course of illness to identify whether a given treatment is really working.
Comments
2 Responses to “The N-acetylcysteine Story: A New Potential Therapy for Bipolar Illness and Substance Abuse”
Leave a Reply


Thank you for this very,very helpfulL information. I have been reading and trying to understand this dreadfull illness for years. I have two sibblings in my country, Nigeria who have been stricking with this illness. My Mother died, then my older sister and lastly, my dad. All this while, my two bipolar sibblings were not present at their funeral. My families (both here and there) have suffered the stigma and shame that’s associated with this illness. First they say, it is a curse, then they blame it on my parents and now, they are looking at me as a possible suspect and peharps do away with me too. One of my bipolar sibling is now receiving the injection and if we can afford it, the pill is added too. My other sibbling refuses to comply. I NEED HELP PLEASE!!! I have no more money to care for these two.
[…] This post was mentioned on Twitter by TheBiPolarBearMD, Tom Kelly. Tom Kelly said: The N-acetylcysteine Story: A New Potential Therapy for Bipolar Illness and Substance Abuse check it out at https://bipolarnews.org/?p=40 […]