Selective Erasure Of Cocaine Memories In A Subset Of Amygdala Neurons
We have written before about the link between memory and both fear and addiction. At a recent scientific meeting, researchers discussed attempts to erase cocaine-cue memories in mice.
In articles published in Science in 2007 and 2009, Han et al. showed that about 20% of neurons in the lateral amygdala of mice were involved in the formation of a fear memory, and that selective deletion of these neurons could erase the fear memory. Using the same methodology, Josh Sullivan et al. identified neurons that were active in the mouse brain during cocaine conditioning. Amygdala activity showed that the mice preferred an environment where they received cocaine to an environment where they didn’t. The researchers noticed increased cyclic AMP, a messenger that led to increased production of calcium responsive element binding protein (CREB). When the researchers targeted the neurons in the lateral amygdala that were overexpressing CREB, they found that selective destruction of the overexpressing neurons disrupted the cocaine-induced place preference.
The research team further documented this effect by temporarily, rather than permanently, knocking out neuronal function. They could reversibly turn off neurons with an inert compound that promotes neuronal inhibition. Silencing the neurons that were overexpressing CREB before the conditioned place preference testing also limited cocaine-induced place preference memory.
Editor’s Note: While this type of intervention is not feasible in humans with cocaine addiction, these data do shed more light on the mechanisms behind cocaine conditioning.
We have written before that if extinction training to break a cocaine habit or neutralize a learned fear is performed within the brain’s memory reconsolidation window (five minutes to one hour after memory recall), it can induce long-lasting alterations in cocaine craving or conditioned fear.
It is possible that properly timed extinction of cocaine- or fear-conditioned memories might work similarly to the selective silencing of neurons that was carried out in the mice using a drug that inhibited CREB-activated neurons. Determining the commonalities between these ways of eliminating conditioned memories could lead to a whole new set of psychotherapeutic approaches to anxiety disorder, addictions, and other pathological habits.
The Amygdala Plays a Role in Habitual Cocaine Seeking
 At a recent scientific meeting, Jennifer E. Murray et al. presented findings about the amygdala’s role in habitual cocaine seeking. The amygdala is the part of the brain that makes associations between a stimulus and a response. When a person begins using cocaine, a signal between the amygdala and the ventral striatum (also known as the nucleus accumbens), the brain’s reward center, creates a pleasurable feeling for the person. The researchers found that in mice who have learned to self-administer cocaine, as an animal progresses from intermittent use to habitual use, the amygdala connections shift away from the ventral striatum toward the dorsal striatum, a site for motor and habit memory. If amygdala connections to the dorsal striatum are severed, the pattern of compulsive cocaine abuse does not develop.
At a recent scientific meeting, Jennifer E. Murray et al. presented findings about the amygdala’s role in habitual cocaine seeking. The amygdala is the part of the brain that makes associations between a stimulus and a response. When a person begins using cocaine, a signal between the amygdala and the ventral striatum (also known as the nucleus accumbens), the brain’s reward center, creates a pleasurable feeling for the person. The researchers found that in mice who have learned to self-administer cocaine, as an animal progresses from intermittent use to habitual use, the amygdala connections shift away from the ventral striatum toward the dorsal striatum, a site for motor and habit memory. If amygdala connections to the dorsal striatum are severed, the pattern of compulsive cocaine abuse does not develop.
Editor’s Note: These data indicate that the amygdala is involved in cocaine-related habit memory, and that the path of activity shifts from the ventral to the dorsal striatum as the cocaine use becomes more habit-based—automatic, compulsive, and outside of the user’s awareness.
As we’ve reviewed before, the amygdala also plays a role in context-dependent fear memories, such as those that occur in post-traumatic stress disorder (PTSD). The process of retraining a person with PTSD to view a stimulus without experiencing fear is called extinction training. A study by Agren et al. published in Science in 2012 demonstrated that when extinction training of a learned fear took place within the brain’s memory reconsolidation window (five minutes to one hour after active memory recall), the training was sufficient to not only “erase the conditioned fear memory trace in the amygdala, but also decrease autonomic evidence of fear as revealed in skin conductance changes in volunteers.”
The preclinical data presented by Murray and colleagues suggest the possibility that amygdala-based habit memory traces could also be revealed via functional magnetic resonance imaging (fMRI) in subjects with cocaine addiction. Attempts at extinction of cocaine craving, if administered within the memory reconsolidation window, might likewise be able to erase the cocaine addiction/craving memory trace, as Xue et al. reported in Science in 2012.
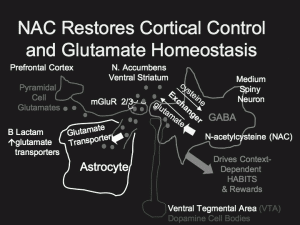
The N-acetylcysteine Story: A New Potential Therapy for Bipolar Illness and Substance Abuse
N-acetylcysteine (NAC), a readily available substance from health food stores, is able to reestablish glutamate homeostasis (regulation and balance) in the reward area of brain (the nucleus accumbens), reported Peter Kalivas of the University of South Carolina at the “Staging neuropsychiatric disorders: Implications for idiopathogenesis and treatment” meeting in Mojacar, Spain this past November. Kalivas reported that NAC appears to be effective across a spectrum of addictions, including cocaine, heroin, alcohol, cigarette smoking, and gambling.
Even more remarkably, NAC also appears to have positive effects in placebo-controlled studies in the treatment of patients with bipolar illness, report Mike Berk and colleagues, who are studying the same substance in Australia. Compared with placebo, patients taking adjunctive NAC showed improvement in all outcome measures, especially depression, after 3 and 6 months. In another article, also published in Biological Psychiatry in 2008, Berk’s research group demonstrated that NAC improved some negative symptoms of schizophrenia. NAC has also shown positive effects in trichotillomania and on nail-biting, suggesting that it has a variety of potential clinical uses in conditions associated with pathological compulsive behavioral patterns.