Deep TMS May Improve Treatment-Resistant Bipolar Depression
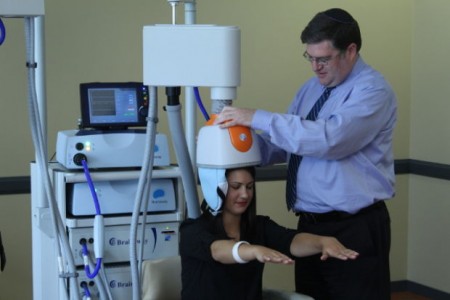
 Deep transcranial magnetic stimulation (dTMS) is a non-invasive treatment that has been shown to be effective in unipolar depression. It consists of a helmet fitted to the head, which uses magnetic coils to create an electric field in a desired brain region.
Deep transcranial magnetic stimulation (dTMS) is a non-invasive treatment that has been shown to be effective in unipolar depression. It consists of a helmet fitted to the head, which uses magnetic coils to create an electric field in a desired brain region.
A 2017 double-blind randomized study by Diego F. Taveres and colleagues in the journal Neuropsychopharmacology found that 20 sessions of dTMS targeting the left dorsolateral prefrontal cortex produced greater improvement in bipolar depression over 4 weeks of treatment than the same number of sham sessions in which participants wore a helmet that delivered similar sounds and scalp sensations without the electrical effects to the brain. The participants had treatment-resistant bipolar depression that was being treated with medication.
However, dTMS’ effects were not significantly different from those of the sham over four additional weeks of follow-up, nor were remission rates significantly different across the two groups. Out of 50 participants, seven dropped out of the study—two from the sham group, and five from the active dTMS group. But there were no occasions on which a participant switched into mania following treatment.
This study suggests that dTMS has the potential to more rapidly improve treatment-resistant bipolar depression as well as unipolar depression.
Deep Transcranial Magnetic Stimulation Safe and Effective in Major Depression
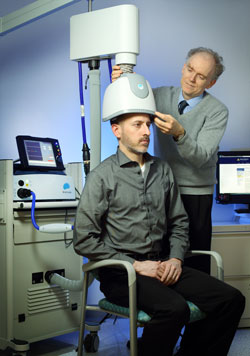
Repeated transcranial magnetic stimulation is a non-invasive procedure that has been approved for the treatment of severe depression since 2008. In rTMS treatment, a figure-8–shaped electromagnetic coil is placed against the forehead and magnetic pulses that can penetrate the scalp are converted into small electrical currents that stimulate neurons in the brain up to 1.5 cm deep. More recently, in 2013, the Federal Drug Administration approved a device with an H-shaped coil that delivers deep transcranial magnetic stimulation (dTMS). It can stimulate a wider area, and up to 8 cm deep.
Y. Levkovitz and colleagues have published the first double-blind randomized controlled multicenter trial of dTMS, reporting in the journal World Psychiatry that the intervention was effective and safe in patients who had not responded to antidepressant medication.
The study included 212 patients aged 22–68 years. All participants had failed to respond to one to four antidepressants or had not been able to tolerate the side effects of at least two antidepressants during their current episode of depression. The patients were randomized to receive either a sham treatment or 18 Hz dTMS over the prefrontal cortex acutely for four weeks and biweekly for 12 weeks for a total of 20 sessions.
The patients who received dTMS showed significantly greater improvement in symptoms than those who received the sham treatment, with a moderately large effect size of 0.76. Response and remission rates were also better in those who received dTMS. Response rates were 38.4% for the dTMS group versus 21.4% in the sham group. Remission rates were 32.6% for the dTMS group and 14.6% for the sham group. These difference in response remained stable during the three months of the study.
Side effects were minor except for a seizure that occurred when the protocol for the treatment was breached.