Treatment Approaches to Childhood-Onset Treatment-Resistant Bipolar Disorder
Dear readers interested in the treatment of young children with bipolar disorder and multiple other symptoms: In 2017, BNN Editor Robert M. Post and colleagues published an open access paper in the journal The Primary Care Companion for CNS Disorders titled “A Multi-Symptomatic Child: How to Track and Sequence Treatment.” The article describes a single case of childhood-onset bipolar disorder shared with us via our Child Network, a research program in which parents can create weekly ratings of their children’s mood and behavioral symptoms, and share the long-term results in graphic form with their children’s physicians.
Here we summarize potential treatment approaches for this child, which may be of use to other children with similar symptoms.
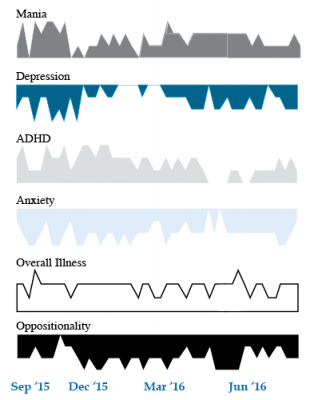
We present a 9-year-old girl whose symptoms of depression, anxiety, attention-deficit hyperactivity disorder (ADHD), oppositional behavior, and mania were rated on a weekly basis in the Child Network under a protocol approved by the Johns Hopkins School of Medicine Institutional Review Board. The girl, whose symptoms were rated consistently for almost one year, remained inadequately responsive to lithium, risperidone, and several other medications. We describe a range of other treatment options that could be introduced. The references for the suggestions are available in the full manuscript cited above, and many quotes from the original article are reprinted here directly.
As illustrated in the figure below, after many weeks of severe mania, depression, and ADHD, the child initially appeared to improve with the introduction of 4,800 micrograms per day of lithium orotate (a more potent alternative to lithium carbonate that is marketed as a dietary supplement), in combination with 1 mg per day of guanfacine, and 1 mg per day of melatonin.
Despite continued treatment with lithium orotate (up to 9,800 micrograms twice per day), the patient’s oppositional behavior worsened during the period from November 2015 to March 2016, and moderate depression re-emerged in April 2016. Anxiety was also generally less severe from December 2015 to July 2016, and weekly ratings of overall illness remained in the moderate severity range (not illustrated).
In June 2016, the patient began taking risperidone (maximum dose 1.7 mg/day) instead of lithium, and her mania improved from moderate to mild. There was little change in her moderate but fluctuating depression ratings, but her ADHD symptoms got worse.
The patient had been previously diagnosed with bipolar II disorder and anxiety disorders including school phobia, generalized anxiety disorder, and obsessive compulsive disorder.
Given the six weeks of moderate to severe mania that the patient experienced in October and November 2015, she would meet criteria for a diagnosis of bipolar I disorder.
Targeting Symptoms to Achieve Remission
General treatment goals would include: mood stabilization prior to use of ADHD medications, a drug regimen that maximizes tolerability and safety, targeting of residual symptoms with appropriate medications supplemented with nutraceuticals, recognition that complex combination treatment may be necessary, and combined use of medications, family education, and therapy.
Mood Stabilizers and Atypical Antipsychotics to Maximize Antimanic Effects
None of the treatment options in this section are approved by the US Food and Drug Administration for use in children under 10 years of age, so all of the suggestions are “off label.” Further, they may differ from what other investigators in this area of medicine would suggest, especially since evidence-based medicine’s traditional gold standard of randomized placebo-controlled clinical trials is impossible to apply here, given the lack of research in children with bipolar disorder.
As we share in the original article, reintroducing lithium alongside risperidone could be effective, as “combinations were more effective than monotherapy in a study [by] Geller et al. (2012), especially when they involved an atypical antipsychotic such as risperidone. This might include the switch from lithium orotate to lithium carbonate,” the typical treatment for bipolar disorder, on which more research has been done. “Combinations of lithium and valproate were also more effective than either [drug alone]…in the studies of Findling et al. (2006),” and many patients needed stimulants in addition.
“Most children also needed combinations of mood stabilizers (lithium, carbamazepine, valproate) in the study [by] Kowatch et al. (2000).” In a 2017 study by Berk et al. of patients hospitalized for a first mania, randomization to lithium for one year was more effective than quetiapine on almost all outcome measures.
Targeting ADHD
“[The increased] severity of [the child’s] ADHD despite improving mania speaks to the…utility of adding a stimulant to the regimen that already includes…guanfacine,” which is a common non-stimulant treatment for ADHD. “This would be supported by the data of Scheffer et al. (2005) that stimulant augmentation for residual ADHD symptoms does not [worsen] mania, and that the combination of a stimulant and guanfacine may have more favorable effects than stimulants alone.”
However, the consensus in the field is that mood stabilization should be achieved first, before low to moderate (but not high) doses of stimulants are added. “Thus, in the face of an inadequate response to the lithium-risperidone combination in this child, stimulants could be deferred until better mood stabilization was achieved.”
Other Approaches to Mood Stabilization and Anxiety Reduction
“The anticonvulsant mood stabilizers (carbamazepine, lamotrigine, and valproate) each have considerable mood stabilizing and anti-anxiety effects, at least in adults with bipolar disorder. With inadequate mood stabilization of this patient on lithium and risperidone, we would consider the further addition of lamotrigine.
Lamotrigine appears particularly effective in adults with bipolar disorder who have a personal history and a family history of anxiety (as opposed to mood disorders), and it has positive open data in adolescents with bipolar depression and in a controlled study of maintenance (in teenagers 13–17, but not in preteens 10–12) (Findling et al. 2015). With better mood stabilization, anxiety symptoms usually diminish…, and we would pursue these strategies [instead of using] antidepressants for depression and anxiety in young children with bipolar disorder.”
“Carbamazepine appears to be more effective in adults with bipolar who have [no] family history of mood disorders,” unlike lithium, which seems to work better in people who do have a family history of mood disorders.
“While the overall results of oxcarbazepine in childhood mania were negative, they did exceed placebo in the youngest patients (aged 7–12) as opposed to the older adolescents (13–18) (Wagner et al. 2006).
“There are long-acting preparations of both carbamazepine (Equetro) and oxcarbazepine (Oxtellar) that would allow for all nighttime dosing to help with sleep and reduce daytime side effects and sedation. Although data [on] anti-manic and antidepressant effects in adults are stronger for carbamazepine than oxcarbazepine,” there are good reasons to consider oxcarbazepine. First, there is the finding mentioned above that oxcarbazepine worked best in the youngest children. Second, there is a lower incidence of severe white count suppression on oxcarbazepine. Third, it has less of an effect on liver enzymes than carbamazepine. However, low blood sodium levels are more frequent on oxcarbazepine than carbamazepine.
Other Atypical Antipsychotics That May Improve Depression
Management of Unipolar and Bipolar Depression During Pregnancy
 At the Maryland Psychiatric Research Society’s continuing medical education conference in November, Lauren Osbourne, Assistant Director of the Women’s Mood Disorders Clinic at Johns Hopkins Hospital, gave a presentation on the management of mood and anxiety during pregnancy and lactation. She had a number of important ideas for physicians and patients to consider in their decision-making process.
At the Maryland Psychiatric Research Society’s continuing medical education conference in November, Lauren Osbourne, Assistant Director of the Women’s Mood Disorders Clinic at Johns Hopkins Hospital, gave a presentation on the management of mood and anxiety during pregnancy and lactation. She had a number of important ideas for physicians and patients to consider in their decision-making process.
According to Osbourne, 60%-70% of pregnant women with unipolar depression who discontinue their antidepressants relapse. Of those with bipolar disorder who discontinue their mood stabilizers, 85% relapse, while 37% of those who stay on their medications relapse.
Something to consider when deciding whether to continue medication while pregnant is that depression in pregnancy carries its own risks for the fetus. These include preterm delivery, low birth weight, poor muscle tone, hypoactivity, increased cortisol, poor reflexes, and increased incidence of attention deficit hyperactivity disorder (ADHD) and other behavioral disorders.
The placenta makes an enzyme 11-BHSD2 that lowers the stress hormone cortisol in the baby. However, this enzyme is less active in depression, exposing the fetus to higher levels of cortisol.
Thus, the decision about whether to continue medications during pregnancy should consider the risks to the fetus of both the mother’s depression and the mother’s medications.
Most antidepressants are now considered safe during pregnancy. There have been reports of potential problems, but these data are often confounded by the fact that women with more severe depression are more likely to require antidepressants, along with other risk variables such as smoking or late delivery (after 42 weeks). When these are accounted for by using matched controls, the apparent risks of certain antidepressants are no longer significant. This includes no increased risk of persistent pulmonary hypertension, autism, or cardiac malformations.
There may be a possible increased risk of Neonatal Adaption Syndrome (NAS) in the first weeks of life in babies who were exposed to selective serotonin reuptake inhibitor (SSRI) antidepressants in the third trimester. This syndrome presumably results from antidepressant withdrawal, and can include respiratory distress, temperature changes, decreased feeding, jitteriness/irritability, floppiness or rigidity, hypoglycemia, and jaundice. There is not yet a robust literature on the syndrome, but Osbourne suggested that it disappears within 2 weeks of birth.
In her practice, Osbourne prefers to prescribe sertraline, which has the best safety data, along with fluoxetine. Sertraline is also OK for breastfeeding. There is less data on bupropion, but it also appears to be safe during pregnancy. Endocrine and enzyme changes in pregnancy typically cause a 40% to 50% decrease in concentrations of antidepressants, so doses of antidepressants typically must be increased in order to maintain their effectiveness.
Osbourne ranked mood stabilizers for bipolar disorder, from safest to most worrisome. Lamotrigine is safest. There is no evidence linking it to birth defects, but higher doses are required because of increased clearance during pregnancy. Lithium is next safest. There are cardiac risks for one in 1,200 patients, but these can be monitored. Carbamazepine is third safest. One percent of babies exposed to carbamazepine will develop spina bifida or craniofacial abnormalities. Valproate is least safe during pregnancy. Seven to ten percent of babies exposed to valproate will develop neural tube defects, other malformations, or developmental delay, with a mean decrease of 9 IQ points. The atypical antipsychotics all appear safe so far.
Alternatives and Adjuncts to Medications in Pregnancy
A Calculator of Risk for Bipolar Disorder in Youth
Daniella Hafeman of the University of Pittsburgh described a risk calculator for predicting an individual’s risk for bipolar disorder, which is available at www.pediatricbipolar.pitt.edu. Possible factors included in the risk calculation include a parent’s early age of onset of bipolar disorder, mood shifts early in life, a child’s anxiety or depression symptoms, later affective mood shifts, and new onset of subthreshold mania.
Editor’s Note: A “poor man’s” assessment of risk can also be of help to a family or clinician. There are four components. The first is genetic. Having one parent with bipolar disorder is a potent risk factor, and can be further magnified if the other parent also has a mood disorder. If three or more first degree relatives or three or more generations of first degree relatives have a mood disorder, this further increases risk four- to six-fold.
Perinatal vulnerability is another factor. Beyond these genetic vulnerabilities, a history of maternal toxoplasmosis or a viral infection during pregnancy, or the infant being noticeably underweight at birth can contribute to bipolar risk.
Childhood adversity also contributes to vulnerability to early onset of bipolar illness. A history of psychosocial stress in the child’s early years, such as abuse or abandonment, can be an added risk factor.
Prodromal or preliminary symptoms are also a risk factor. The development of an anxiety or depressive disorder, a disruptive behavioral disorder, or a bipolar not-otherwise-specified diagnosis (BP-NOS, used to describe manic symptoms of short duration) further increases risk. In studies by David Axelson and Boris Birmaher, 50% of children with an initial diagnosis of BP-NOS developed full-blown bipolar I or II illness upon several years of followup if there was a family history of bipolar disorder. About one-third converted to full bipolar disorder if there was no family history of bipolar disorder.
Thus, if a child has three or all four types of risk factors, their risk would be substantial. In this case, one might consider attempts at prevention. This could include a good diet rich in omega-3 fatty acids, regular exercise, joining a school sports team, developing good sleep habits, playing a musical instrument, and engaging in something akin to family focused therapy. Family focused therapy emphasizes psychoeducation, good communication skills, and problem solving. Attending to and treating parents’ symptoms and building a support system for both parents and the child can also help.
While these endeavors are not a guarantee to prevent the onset of more severe illness, they are all health-promoting in general and have few downsides.
Making Lithium Treatment More Tolerable For Patients
 In a slideshow at Psychiatric Times, Chris Aiken describes seven ways to improve lithium’s tolerability. Since many researchers, including BNN Editor-in-Chief Robert M. Post, have suggested that lithium should be used more often as a treatment for bipolar disorder, ways of making its side effects more manageable are of great interest. Here we summarize Dr. Aiken’s seven points and add a few perspectives of our own.
In a slideshow at Psychiatric Times, Chris Aiken describes seven ways to improve lithium’s tolerability. Since many researchers, including BNN Editor-in-Chief Robert M. Post, have suggested that lithium should be used more often as a treatment for bipolar disorder, ways of making its side effects more manageable are of great interest. Here we summarize Dr. Aiken’s seven points and add a few perspectives of our own.
Aiken writes that “when it comes to the side effects that matter most to patients—sedation, weight gain, and cognition—lithium’s tolerability ranks right behind lamotrigine.” In fact, lithium plus lamotrigine is an excellent combination, as lithium excels at preventing manias while lamotrigine excels at depression prevention.
Post’s philosophy is that many of lithium’s side effects can be avoided in the first place through judicious dose titration. He suggests gradually increasing dosage, and stopping before side effects become difficult, or reducing a dosage that has already become a problem. The idea is to avoid lithium side effects even if blood levels of lithium remain below clinically therapeutic levels. Post suggests using lithium at whatever dose is not associated with side effects.
Many of lithium’s positive therapeutic effects emerge at low doses, and if this improvement is insufficient, the rest of the needed efficacy can be achieved by adding other medications. As noted above, lamotrigine is a good option for break-through depression, as is lurasidone. For breakthrough mania, the mood stabilizers valproate and carbamazepine or an atypical antipsychotic can be added to lithium.
A little-appreciated option for enhancing lithium’s mood stabilizing effects is nimodipine, a dihydropyridine calcium blocker. It has both antimanic and antidepressant efficacy without lithium’s side effects. Research showed that a year on the combination of lithium and nimodipine was more effective than a year of either drug alone.
If a patient taking lithium experiences a tremor at a dose that is not fully effective, nimodipine can be added in order to lower the lithium dose enough to eliminate the tremor.
Nimodipine specifically blocks the calcium influx gene CACNA1C that has been repeatedly been associated with the vulnerability to bipolar disorder and depression.
If side effects do occur on lithium, they can often be managed. The following suggestions are adapted from Aiken’s article with input from Post. Read more
Dietary Supplements for Autism: Up-to-Date Research
A 2017 review article by Yong-Jiang Li and colleagues in the journal Frontiers in Psychiatry describes the current research on dietary supplements that may help improve symptoms of autism spectrum disorder.
Some of the most promising research was on vitamin D, folinic acid, and sulforaphane. Methyl B12 and digestive enzyme therapy had some positive effects, while gluten- and casein-free diets and omega-3 fatty acids did not seem to help improve autism symptoms.
Vitamin D
Li and colleagues described a randomized, controlled trial of vitamin D in 109 children with autism aged 3 to 10 years. The experimental group received doses of 300 IU/kg of body weight/day, not exceeding 5000 IU/day. By the end of the four-month study, vitamin D levels had significantly increased in the experimental group compared to the control group. Those who received vitamin D also showed significant improvement on all ratings of autism symptoms, which included general scales of autism symptoms and more specialized checklists that capture aberrant behavior and social responsiveness.
Folinic Acid
The review article also described a randomized double-blind placebo-controlled trial of folinic acid in 48 children with autism spectrum disorder and language impairment. Participants received high-dose folinic acid (2 mg/kg/day) or placebo for 12 weeks. Those who received folinic acid, a form of folic acid that can readily be used by the body, showed significant improvements in verbal communication and core autism symptoms compared to those who received placebo. Participants who tested positive for folate receptor alpha autoantibodies (FRAA), which disrupt the transportation of folate across the blood-brain barrier and are common in autism, showed greater improvements from taking folinic acid than those without this abnormality.
Sulforaphane
Sulforaphane is a phytochemical derived from cruciferous vegetables. It can create metabolic effects that resemble those of a fever, which can improve behavioral symptoms of autism. Sulforaphane also fights oxidative stress, inflammation, and DNA damage, which may play roles in autism. Li and colleagues described the first double-blind, placebo-controlled trial of sulforaphane treatment in 29 boys aged 13 to 17 years. The boys who received sulforaphane showed significant improvement in autism-related behavior, especially social interaction and communication, after 18 weeks compared to those who received placebo. Sulforaphane has low toxicity and is well tolerated. Read more
Only Fluoxetine is More Effective Than Placebo for Children and Adolescents with Depression
 In a meta-analysis published in 2016, researchers Andrea Cipriani, Xinyu Zhou, and colleagues reported that many antidepressants are not effective in children and adolescents. Fluoxetine alone was more effective than placebo. Other antidepressants also caused high study drop-out rates compared to placebo.
In a meta-analysis published in 2016, researchers Andrea Cipriani, Xinyu Zhou, and colleagues reported that many antidepressants are not effective in children and adolescents. Fluoxetine alone was more effective than placebo. Other antidepressants also caused high study drop-out rates compared to placebo.
In an article published in the journal The Lancet, Cipriani, Zhou, and colleagues analyzed 34 randomized, controlled clinical trials of antidepressants in children and adolescents. These trials included a total of 5,260 participants and 14 different antidepressants.
The researchers determined that much of the evidence was of a low quality. Only fluoxetine was statistically significantly more effective than placebo. Fluoxetine was also more tolerable to patients than duloxetine or imipramine. Patients who received imipramine, venlafaxine, or duloxetine were more likely to drop out of studies due to adverse events compared to patients who received placebo.
The authors suggest that prescribing antidepressants to children or adolescents may not necessarily be beneficial, and that fluoxetine is probably the best option to consider.
Editor’s Note: It may be best to use caution when prescribing antidepressants to children or adolescents. First, these data that suggest that many antidepressants are ineffective in young people. In addition, depression in children and adolescents may be a sign of bipolar disorder, and antidepressant use may cause activation or switching into mania in vulnerable patients.
While there is a warning about using antidepressants in young people because of the risk of increased suicidal ideation, the actual suicide rate in young populations decreases when these patients take antidepressants and cognitive behavioral therapy. Psychotherapy should be a high priority. Other safe adjunctive approaches might include omega-3 fatty acids, N-acetylcysteine, vitamin D3, and folic acid. Evidence for the efficacy of rTMS in young people is also positive and growing.
Mysteries Remain in the Relationship Between Inflammation and Depression
At the 2017 meeting of the American College of Psychiatrists, researchers Charles L. Raison and Vladimir Maletic gave a plenary lecture on the role of inflammation in depression. Meta-analyses have confirmed that inflammatory markers including Il-1, Il-6, TNF alpha, and CRP are elevated in about 1/3 of depressed patients. However, Raison and Maletic made the point that anti-inflammatory medications are not for everyone. While patients with elevated levels of CRP at baseline responded to an anti–TNF alpha antibody, patients with low CRP values at baseline actually got worse.
Raison and Maletic cited three studies that have also linked CRP to differential response to traditional antidepressants. In unipolar depression, those with low CRP respond well to selective serotonin reuptake inhibitor (SSRI) antidepressants, while those with elevated blood levels of CRP seem to respond better to a dopamine-active antidepressant such as bupropion or a noradrenergic-active antidepressant such as nortriptyline or the serotonin norepinephrine reuptake inhibitor (SNRI) antidepressant duloxetine. Patients with high inflammation at baseline also seem to respond better to intravenous ketamine and oral doses of omega-3 fatty acids.
Studies of animals have suggested that inflammation throughout the body is implicated in depression. Studies in which rodents are repeatedly defeated by larger animals show that these animals have increased inflammation from lymphocites (a type of white blood cells) in the blood, and monocytes (another type of white blood cells) from the bone marrow and spleen. This inflammation can induce depression-like behaviors in the rodents, which is prevented if the inflammatory mechanisms are blocked. These data suggest that depression is not just in the brain—inflammation from all over the body plays an important role.
Read more
Inflammatory Marker CRP Higher in Bipolar Disorder, Particularly Mania
Inflammation has been linked to mood disorders. A 2016 meta-analysis in the journal Lancet Psychiatry described the role of inflammatory marker C-reactive protein (CRP) in bipolar episodes. Researchers led by Brisa S. Fernandes identified 27 studies that measured CRP levels in a total of 2,161 patients with bipolar disorder and 81,932 healthy participants. The researchers determined that compared to healthy controls, people with bipolar disorder have higher levels of CRP. CRP levels were moderately elevated between episodes and during depression, and substantially elevated during episodes of mania.
Editor’s Note: This meta-analysis shows that CRP is linked to bipolar disorder, and the inflammatory burden is highest during mania. It remains to be seen whether anti-inflammatory treatments work best in patients with high CRP levels compared to normal CRP levels.
CRP is also a risk factor for cardiovascular disease, and lithium and other treatments for bipolar disorder probably lower CRP levels.
The same group of researchers previously showed that statins, drugs typically used to lower cholesterol, could help alleviate depression. Since statins have anti-inflammatory effects, they can probably reduce depression risk in addition to lowering cardiovascular risk, as initial studies suggest.
Other drugs with anti-inflammatory effects that may improve depression include the anti-arthritis drug celecoxib and the antibiotic minocycline. The amino acid N-acetylcysteine and omega-3 fatty acids also have anti-inflammatory effects and have been found to improve depression in some studies.
Certain Types of Inflammation and BMI Predict Depression
At the 2016 meeting of the Society of Biological Psychiatry, researcher Femke Lamers and colleagues presented findings from the Netherlands Study of Depression and Anxiety. The inflammatory markers interleukin-6 and CRP were elevated in people with current major depression. These measures were correlated with BMI, a measure of body weight. High levels of interleukin-6 at the beginning of the study predicted who would have a chronic course of illness.
Editor’s Note: Previous studies have found that elevated levels of CRP predicted a future mood episode in people at high risk for bipolar disorder due to a family history of the illness.
These studies suggest that it might be useful to assess levels of these inflammatory markers (CRP, interleukin-1, interleukin-6, and TNF-alpha) in young people who are at high risk for bipolar disorder. Factors that put someone at high risk include a family history of depression or bipolar disorder, a history of adversity in childhood (abuse, neglect, loss of a parent, etc.), and preliminary symptoms.
Several interventions are available that may reduce the likelihood that someone at risk for bipolar disorder will go on to develop the illness. Family interventions such as the Family Focused Therapy developed by researcher David Miklowitz are helpful. In a 2013 study in the Journal of the American Academy of Child and Adolescent Psychiatry, Miklowitz reported that Family Focused Therapy outperformed treatment as usual for youth at risk for bipolar disorder.
Measures of inflammation might provide additional rationale for beginning interventions in youth at high risk for mood disorders. In addition to family interventions, omega-3 fatty acid supplementation is a low-risk option that is supported by some positive data. Since BMI was implicated in the study by Lamers and colleagues, keeping weight under control might also have some benefit.
For adults with depression who want to keep their weight under control, the combination of the antidepressant bupropion XR (150–300mg/day) and naltrexone (50mg/day), an opiate antagonist medication normally used to fight addictions, has been effective.
Meta-Analysis Shows Anti-Inflammatory Treatments Improve Bipolar Depression
 It has been clear for some time that depression and inflammation are linked. This has led researchers to explore a variety of anti-inflammatory agents to treat depression. A meta-analysis of studies examining anti-inflammatory treatments for bipolar depression was published in the journal Bipolar Disorders in 2016.
It has been clear for some time that depression and inflammation are linked. This has led researchers to explore a variety of anti-inflammatory agents to treat depression. A meta-analysis of studies examining anti-inflammatory treatments for bipolar depression was published in the journal Bipolar Disorders in 2016.
Researcher Joshua D. Rosenblat and colleagues identified eight randomized controlled trials that met their criteria for anti-inflammatory treatments of bipolar disorder. These treatments included nonsteroidal anti-inflammatory drugs (NSAIDs such as ibuprofen and aspirin), omega-3 fatty acids, the antioxidant N-acetylcysteine, and pioglitazone (used to treat diabetes). Overall, the anti-inflammatory treatments had a moderate and statistically significant antidepressant effects. No serious side effects were reported, and the anti-inflammatory treatments did not cause a switch into mania in any of the participants.
The diversity of the anti-inflammatory treatments reviewed in this meta-analysis limit the extent to which it can be interpreted, but it is clear that more research on anti-inflammatory treatments for bipolar depression is needed. An open question is whether patients with particularly elevated levels of inflammatory markers in their blood would respond better to these anti-inflammatory treatments.