Dr. Post’s Recommendations For Treating Youth with Bipolar Symptoms
Our Editor-in-Chief, Dr. Robert M. Post, shares his personal recommendations for the treatment of children and adolescents with symptoms of bipolar disorder. Remember: Patients and family members must consult a physician about all information conveyed in the BNN. With the exception of lithium, none of the medications or supplements discussed above have been approved by the US Food and Drug Administration for use in children under 10. The findings reported here are in many cases preliminary and cannot be taken as recommendations based on the short summaries provided here. All treatment decisions must be made in conjunction with a patient’s treating physician, who is solely responsible for initiating any treatment discussed in the BNN or elsewhere.
In symptomatic and functionally impaired children, medication is almost always necessary. Many treating psychiatrists would start with an atypical antipsychotic, since there is clear evidence of the efficacy of such treatments. The side effects profile should be considered, as there is a considerable difference in the degree of weight gain associated with different atypical antipsychotics. The largest weight gains occur with olanzapine and clozapine, intermediate gains occur with aripiprazole and quetiapine, and the least gains occur with ziprasidone and lurasidone (and the latter has the advantage of being approved by the US Food and Drug Administration for the treatment of bipolar depression in children who are 10–17 years old). The addition of the diabetes drug metformin to decrease weight gain in people taking atypical antipsychotics is increasingly common.
The addition of an anticonvulsant medication (such as lamotrigine, carbamazepine/oxcarbazepine, or valproate) or the mood stabilizer lithium may be needed, as multiple studies indicate that combination treatment is typically needed in children (as in adults) to achieve a more complete response or remission.
Interestingly, oxcarbazepine was effective in younger but not older children with mania in a previous placebo-controlled study by Karen D. Wagner and colleagues published in the American Journal of Psychiatry in 2006.
Conversely, in a 2015 article in the journal JAACAP, researcher Robert Findling reported that in a placebo-controlled study of lamotrigine, 13–17-year-olds responded better than 10–12-year-olds.
Lithium treatment deserves consideration in children with classical presentations of bipolar disorder and those who have family members who have responded well to lithium treatment.
Lithium has the benefit of improving the white matter abnormalities seen in the brains of patients with early-onset bipolar disorder. Hafeman and colleagues reported in a 2019 article that children with bipolar disorder who were treated with lithium had better long-term results upon follow up than those treated with atypical antipsychotics or anticonvulsants.
There is much less scientific consensus about other adjunctive treatments for young people with additional bipolar symptoms and comorbidities, but this editor often uses several. Vitamin D3 is often low in children with psychiatric illness, and may improve mood and cognition.
The antioxidant N-acetylcysteine (NAC) helps depression, anxiety, and irritability, and is effective at treating habit-related behaviors such as trichotillomania (compulsive hair-pulling), obsessive-compulsive disorder (OCD), and drug use, including specifically reducing marijuana use in adolescents. A typical dose is 500–600 mg capsules, one capsule twice a day for one week, then two capsules in the morning and two in the evening thereafter.
Folate or folic acid may enhance antidepressant effects and those of lithium. In patients who have a particular low-functioning variant of a gene known as MTHFR, L-methylfolate is required instead of folate.
The widely-used supplement acetyl-L-carnitine (ALC) is poorly studied in children, but deserves consideration as a supplemental treatment for patients with histories of childhood adversity. In adults with depression, blood levels of ALC may be low, particularly in those with an early onset of bipolar symptoms and a history of childhood adversity (see a 2018 article by Carla Nasca in the journal PNAS). There is a modicum of evidence that ALC produces antidepressant effects in adults. ALC may also sensitize insulin receptors and normalize blood pressure.
There is increasing evidence of the role of inflammation in depression, mania, post-traumatic stress disorder (PTSD), and schizophrenia. Checking for abnormalities in inflammatory markers in the blood (especially Il-6 and CRP) may point the way to appropriate therapy with anti-inflammatory drugs such as minocycline (100 mg twice a day) or celecoxib (200 mg twice a day) in patients who do not respond fully to first-line medications.
Treating Bipolar Depression in an Adolescent
At the 2019 meeting of the International Society for Bipolar Disorders, researcher Ben Goldstein discussed a case of a 15-year-old with bipolar depression and his recommended treatments for the adolescent. Goldstein endorsed the use of an atypical antipsychotic such as lurasidone, and perhaps also quetiapine. Goldstein noted 2015 findings from researcher Robert Findling that lamotrigine was significantly more effective than placebo in adolescents 13–18 years old, but was not effective in those aged 10–12.
(In adults, researcher John Geddes and colleagues found that in patients with an inadequate antidepressant response to quetiapine, the addition of lamotrigine was more effective than adding a placebo, both acutely and in long-term follow-up. The only caveat was that lamotrigine was less effective in those who were also being treated with folate.)
Editor’s Note: Some other treatments could augment the effects of the regimen proposed by Goldstein, including lithium and the antioxidant N-acetylcysteine, which, it should be noted, takes more than eight weeks to become effective. Vitamin D3 could also be considered, as it is often low in children with psychiatric disorders. One treatment that went unmentioned at the meeting was repeated transcranial magnetic stimulation, or rTMS, which is effective and well-tolerated in adolescents with depression.
For patients with more rapidly cycling bipolar disorder and only partial response to medications, the combination of the ‘three Ls’ (lurasidone, lamotrigine, and lithium) could have considerable appeal, given that each drug is from a different class of medications, has a different mechanism of action, targets a different mood phase, and is relatively well-tolerated both alone and in combination with other drugs.
Preventing Illness in the Offspring of a Parent with Bipolar Disorder
A 2018 article by researcher Robert Freedman and colleagues in the American Journal of Psychiatry reported that prenatal nutritional supplements can reduce mental illness in at-risk offspring. The article made a good case for supplementation with folate, phosphatidylcholine, and vitamins A and D.
Here we describe some additional ways to minimize risk of mental illness in children who are at risk for bipolar disorder or other mental illnesses.
Some efforts at prevention can begin even before a child is conceived. Avoiding smoking or drinking alcohol and maintaining a nutritious diet to prevent inflammation and excessive weight gain before conception could reduce adverse epigenetic effects on the offspring. Epigenetics refers to environmental influences on gene transcription. The impact of life experiences such as a mother or father’s substance use is not registered in their child’s DNA sequence, but can influence the structure of the child’s DNA or its packaging.
Maternal good health and wellbeing during pregnancy has also been shown to improve neonatal health and functioning.
Once a child is born, they can be encouraged in healthy habits, including a nutritious diet, good sleeping habits, regular vigorous exercise, and mindfulness/meditation training (which pediatric psychiatrist James Hudziak has suggested should be universal).
For a child who is beginning to develop mood or behavioral symptoms, more intensive intervention may be prudent. Research supports the effectiveness of family interventions such as family-focused therapy (FFT) for youth with depression, cyclothymia, or bipolar disorder not otherwise specified (BP-NOS) and a family history of bipolar disorder. Researcher David J. Miklowitz described the effects of this intervention in a 2013 article in the Journal of the American Academy of Child and Adolescent Psychiatry.
Depression in children 3 to 6 years of age is as common as depression in older children (with rates around 1–2%), and robust improvements have been observed when families engage in parent child interaction therapy (PCIT) with a focus on emotional development. In PCIT, parents are coached while interacting with their children and encouraged to establish warm interactions while setting appropriate limits. In a study by Joan L. Luby and colleagues published in the American Journal of Psychiatry in 2018, using PCIT modified to include an emotional development component improved depression and associated symptoms in children aged 3 to 11, and it also improved mothers’ mood and behavior. Read more
Prenatal Prevention of Psychiatric Illness with Nutritional Supplements
 In a 2018 article in the American Journal of Psychiatry, researcher Robert Freedman and colleagues shared the results of a systematic review of data on nutritional supplements during pregnancy for the primary prevention of psychiatric illness in the child. Freedman and colleagues concluded that the evidence is robust that prenatal folic acid supplementation plus multivitamins not only can prevent birth defects such as cleft palate, spina bifida, and microcephaly, but also social withdrawal, decreased attention, and aggression at age 18 months. They wrote, “Supplements of up to 4 mg [of folic acid] before 12 weeks gestation have been found to be safe and effective.”
In a 2018 article in the American Journal of Psychiatry, researcher Robert Freedman and colleagues shared the results of a systematic review of data on nutritional supplements during pregnancy for the primary prevention of psychiatric illness in the child. Freedman and colleagues concluded that the evidence is robust that prenatal folic acid supplementation plus multivitamins not only can prevent birth defects such as cleft palate, spina bifida, and microcephaly, but also social withdrawal, decreased attention, and aggression at age 18 months. They wrote, “Supplements of up to 4 mg [of folic acid] before 12 weeks gestation have been found to be safe and effective.”
The effects of omega-3 fatty acid supplementation depended on when the supplements were taken. Taking omega-3 fatty acid supplements early in pregnancy was linked to an increase in schizophrenia and more symptoms of attention deficit hyperactivity disorder (ADHD) in the offspring. However, supplementation after 20 weeks of pregnancy decreased preterm delivery, low birth weight, and asthma.
As of 2017, choline supplementation during pregnancy is recommended by the American Medical Association. Their recommendation is based on research in which the choline precursor phosphatidylcholine (5,000-6,300 mg/day) was given to mothers beginning in the 18th week of pregnancy and continued in the newborn for two weeks to three months after birth in the form of 100mg of liquid phosphatidylcholine. This supplementation regimen normalized the P50 auditory evoked potential, a measure of inhibitory sensory gating that is abnormal in patients with schizophrenia and bipolar disorder and infants whose parents had psychosis, depression, or smoked (all risk factors for a later diagnosis of schizophrenia).
Healthy individuals show a reduced response to an auditory cue when it is repeated 50 milliseconds after the initial cue. In people with schizophrenia, response to the repeated cue is not suppressed. Not only did the P50 auditory evoked potential normalize with phosphatidylcholine supplementation, but at 3.5 years of age, those who received phosphatidylcholine supplements in utero and as newborns had fewer problems with attention and social interactions. The findings were even more robust in those with the CHRNA7 genotype (a genetic variation in the alpha 7 nicotinic receptor), which is a risk factor for schizophrenia.
Supplementation with vitamins A and D during gestation also decreased the risk for schizophrenia and autism spectrum disorders in offspring. Recommendations include Vitamin D at doses of 600 to 4,000 IU for pregnant mothers and 400 to 1,000 IU for infants. Because of potential toxicity, vitamin A should be limited to 8,000 units from diet and supplements combined. (Supplements typically contain 2,500 units.)
While there are some methodological limitations to the findings, Freedman and colleagues conclude, “As part of comprehensive maternal and fetal care, prenatal nutrient interventions should be further considered as uniquely effective first steps in decreasing risk for future psychiatric and other illnesses in newborn children.”
Editor’s Note: Given the high risk of psychiatric illness (74%) in the offspring of a parent with bipolar disorder and the finding of abnormal P50 auditory evoked potential in patients with bipolar disorder, the recommended nutritional supplements should be given special consideration during gestation of a child who has a parent with bipolar disorder. According to the 2018 article by Freedman and colleagues, this would include folate, phosphatidylcholine, vitamin A and vitamin D.
Antioxidant Supplement Coenzyme Q10 Looks Promising for Bipolar Depression
Coenzyme Q10 (CoQ10) is an antioxidant that occurs naturally in the human body, but its levels decline with age, medical illness, and depression. In a randomized, controlled trial that was published in the Journal of Clinical Psychopharmacology in 2018, researcher Maryam Mehrpooya and colleagues found that adding coenzyme Q10 supplements to a treatment regimen improved bipolar depression compared to adding placebo.
The pathophysiology of bipolar disorder involves mitochondrial dysfunction, oxidative stress, and inflammation, and coenzyme Q10 can affect all of these pathways. It is also neuroprotective, and may help prevent the degeneration of neurons in people with Alzheimer’s, Parkinson’s, or Huntington’s diseases.
The study included a final total of 69 participants who were randomly assigned to receive either 200 mg/day of coenzyme Q10 supplements or placebo in addition to their normal treatment regimen, which had been stable for at least two months at the time of the study. Participants’ bipolar depression was rated at the beginning of the study, after four weeks, and after eight weeks. At the eight-week mark, coenzyme Q10 showed a statistically significant benefit over placebo with a large effect size. Three participants who received coenzyme Q10 experienced full remission of their depression, and 72% of those in the coenzyme Q10 group improved compared to only 12% of those who received placebo.
The study had some limitations. It was small, and twenty participants dropped out of the study before its completion, which may have inflated the findings.
Previous research found that coenzyme Q10 had benefits in specific populations. In two non-blind studies (studies in which participants know that they are receiving the treatment in question rather than possibly a placebo), 29 older patients with bipolar disorder improved when taking 800 mg to 1200 mg/day of coenzyme Q10. A randomized, controlled trial of coenzyme Q10 in people with multiple sclerosis and depression found that 500 mg/day reduced fatigue symptoms and depression. Coenzyme Q10 has also improved well-being and energy in small, controlled trials in people with breast cancer, Gulf War veterans, and elderly populations.
Taking coenzyme Q10 is low-risk. It had no adverse effects in the study by Mehrpooya and colleagues. Gastrointestinal reactions are possible, but can be managed by taking coenzyme Q10 with food and spreading out dosing throughout the day. Insomnia is also possible, but is less likely when coenzyme Q10 is taken early in the day. One effect to note is that coenzyme Q10 can interact badly with the blood-thinner warfarin.
Editor’s Note: The study by Mehrpooya and colleagues is interesting. Another antioxidant, N-acetylcysteine (NAC), also took 2 months to work in trichotillomania and bipolar depression, so patients should be warned not to expect a quick response with either coenzyme Q10 or NAC. Other potentially useful supplements include: Vitamin D3 (1500–5000 IU/day), folate or L-methylfolate, and acetyl-L-carnitine. Acetyl-L-carnitine may work more quickly, based on its presumed mechanism (increasing the production of the inhibitory metabotrophic glutamate receptor mGluR-2, which inhibits glutamate release).
Supplements for the Treatment of Schizophrenia
At the 2018 meeting of the North Carolina Psychiatric Association, researcher Karen Graham reviewed evidence for adjunctive treatments that may help treat schizophrenia when added to antipsychotic medications.
Graham endorsed omega-3-fatty acids, saying that they may delay the conversion to schizophrenia in young people at high risk for the illness. Data in chronic schizophrenia are more equivocal.
Data on the effects of vitamin D3 in schizophrenia are mixed, but D3 is often low in patients with psychotic disorders, and supplementation with vitamin D3 in the general population has been associated with decreases in cancer and all-cause mortality.
Graham indicated that in three studies vitamin B6 (pyridoxine) decreased tardive dyskinesia, a side effect of antipsychotic medication that is characterized by repetitive or jerky involuntary movements of the face and body. B6 also reduced the severity of akathisia or restless legs, which is comparable to the effects of 40mg/day of the beta blocker drug propranolol. Graham recommended a dose of 300mg/day of B6 that could be increased up to 600mg twice per day. The onset of effects usually begins by week three, and the cost ranges from 25 to 80 cents per day.
The antioxidant supplement N-acetylcysteine (NAC) may also help. Graham described six studies that found NAC had positive effects on negative symptoms (apathy, blunted emotions, etc.) and/or cognition in patients with schizophrenia. The dosage in these studies was usually 2 grams/day for 24 weeks. The cost was 50 cents per day.
Two 8-week trials of L-theanine (an amino acid found in green and black tea) at doses of 400mg/day improved negative symptoms and anxiety in 40 patients with schizophrenia. The rationale for the study was that L-theanine increases inhibitory neurotransmitters, modulates the amino acid 5-HTP and the neurotransmitter dopamine, increases brain-derived neurotrophic factor (BDNF), and may be neuroprotective after a heart attack or a traumatic brain injury. The cost is 40 cents per day.
Graham reported that the supplement ginkgo biloba produced significant improvement in negative symptoms and total symptoms in eight clinical trials that included a total of 1,033 patients with schizophrenia. Doses ranged from 240 to 360 mg/day. These supplements (usually extracted from leaves of the ginkgo tree) have not been found to have many side effects, but they can reportedly increase post-operative bleeding. Gingko biloba supplements cost 20 to 80 cents per day. There is also at least one positive study of ginkgo biloba in tardive dyskinesia.
Three of four studies of cannabidiol in schizophrenia have been positive (at doses of 600, 800, and 1,000 mg/day in studies that lasted four to six weeks). There are now six additional ongoing studies listed on the website clinicaltrials.gov. There is little of this diol component in regular marijuana, and the cost of pure cannabidiol is unfortunately an exorbitant $60 to $100/day.
There is a positive controlled study of the herb ashwagandha in 66 patients with schizophrenia.
Not included in Dr. Graham’s review was the prenatal treatment of women with phosphatidylcholine (900mg/day) followed by supplements in the newborn, which normalized an aspect of sensory gating known as P50 in patients with schizophrenia. Healthy individuals show a reduced response to an auditory cue when it is repeated 50 milliseconds after the initial cue. In people with schizophrenia, response to the repeated cue is not suppressed. This has been suggested by researchers Robert Freedman and Randal G. Ross in a 2015 article in the Shanghai Archives of Psychiatry as a possible primary preventive approach to schizophrenia.
Pregnant women in their second and third trimesters should at least consume foods high in choline, especially if the fetus is at high risk for schizophrenia because of a family history of schizophrenia.
Beef liver is very high in choline, providing 420mg per slice. Other animal products provide significant choline, such as eggs (120 mg/egg), beef (90mg/100g), chicken liver (85mg/liver), fish (85mg/100g), bacon (35mg/strip) or other pork, chicken (67mg/100g). Tofu (36mg/half cup) and cereal (22mg/half cup) are also sources of choline.
Foods High in Choline
| Beef liver | 1 slice | 420mg choline; |
| Egg | 1 egg | 120; |
| Beef | 100 gm | 90; |
| Chicken liver | 1 liver | 85; |
| Fish | 100 gm | 85; |
| Bacon or pork | 2 strips bacon | 70; |
| Chicken | 100 gm | 67; |
| Tofu | 120 ml (0.5 cup) | 36; |
| Cereal | 120 ml (0.5 cup) | 22 |
Treatment Approaches to Childhood-Onset Treatment-Resistant Bipolar Disorder
Dear readers interested in the treatment of young children with bipolar disorder and multiple other symptoms: In 2017, BNN Editor Robert M. Post and colleagues published an open access paper in the journal The Primary Care Companion for CNS Disorders titled “A Multi-Symptomatic Child: How to Track and Sequence Treatment.” The article describes a single case of childhood-onset bipolar disorder shared with us via our Child Network, a research program in which parents can create weekly ratings of their children’s mood and behavioral symptoms, and share the long-term results in graphic form with their children’s physicians.
Here we summarize potential treatment approaches for this child, which may be of use to other children with similar symptoms.
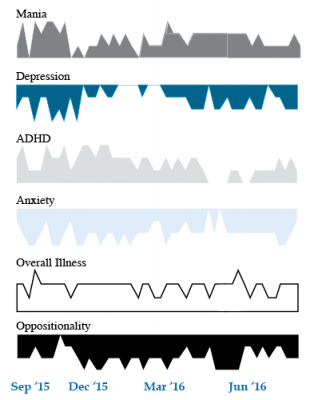
We present a 9-year-old girl whose symptoms of depression, anxiety, attention-deficit hyperactivity disorder (ADHD), oppositional behavior, and mania were rated on a weekly basis in the Child Network under a protocol approved by the Johns Hopkins School of Medicine Institutional Review Board. The girl, whose symptoms were rated consistently for almost one year, remained inadequately responsive to lithium, risperidone, and several other medications. We describe a range of other treatment options that could be introduced. The references for the suggestions are available in the full manuscript cited above, and many quotes from the original article are reprinted here directly.
As illustrated in the figure below, after many weeks of severe mania, depression, and ADHD, the child initially appeared to improve with the introduction of 4,800 micrograms per day of lithium orotate (a more potent alternative to lithium carbonate that is marketed as a dietary supplement), in combination with 1 mg per day of guanfacine, and 1 mg per day of melatonin.
Despite continued treatment with lithium orotate (up to 9,800 micrograms twice per day), the patient’s oppositional behavior worsened during the period from November 2015 to March 2016, and moderate depression re-emerged in April 2016. Anxiety was also generally less severe from December 2015 to July 2016, and weekly ratings of overall illness remained in the moderate severity range (not illustrated).
In June 2016, the patient began taking risperidone (maximum dose 1.7 mg/day) instead of lithium, and her mania improved from moderate to mild. There was little change in her moderate but fluctuating depression ratings, but her ADHD symptoms got worse.
The patient had been previously diagnosed with bipolar II disorder and anxiety disorders including school phobia, generalized anxiety disorder, and obsessive compulsive disorder.
Given the six weeks of moderate to severe mania that the patient experienced in October and November 2015, she would meet criteria for a diagnosis of bipolar I disorder.
Targeting Symptoms to Achieve Remission
General treatment goals would include: mood stabilization prior to use of ADHD medications, a drug regimen that maximizes tolerability and safety, targeting of residual symptoms with appropriate medications supplemented with nutraceuticals, recognition that complex combination treatment may be necessary, and combined use of medications, family education, and therapy.
Mood Stabilizers and Atypical Antipsychotics to Maximize Antimanic Effects
None of the treatment options in this section are approved by the US Food and Drug Administration for use in children under 10 years of age, so all of the suggestions are “off label.” Further, they may differ from what other investigators in this area of medicine would suggest, especially since evidence-based medicine’s traditional gold standard of randomized placebo-controlled clinical trials is impossible to apply here, given the lack of research in children with bipolar disorder.
As we share in the original article, reintroducing lithium alongside risperidone could be effective, as “combinations were more effective than monotherapy in a study [by] Geller et al. (2012), especially when they involved an atypical antipsychotic such as risperidone. This might include the switch from lithium orotate to lithium carbonate,” the typical treatment for bipolar disorder, on which more research has been done. “Combinations of lithium and valproate were also more effective than either [drug alone]…in the studies of Findling et al. (2006),” and many patients needed stimulants in addition.
“Most children also needed combinations of mood stabilizers (lithium, carbamazepine, valproate) in the study [by] Kowatch et al. (2000).” In a 2017 study by Berk et al. of patients hospitalized for a first mania, randomization to lithium for one year was more effective than quetiapine on almost all outcome measures.
Targeting ADHD
“[The increased] severity of [the child’s] ADHD despite improving mania speaks to the…utility of adding a stimulant to the regimen that already includes…guanfacine,” which is a common non-stimulant treatment for ADHD. “This would be supported by the data of Scheffer et al. (2005) that stimulant augmentation for residual ADHD symptoms does not [worsen] mania, and that the combination of a stimulant and guanfacine may have more favorable effects than stimulants alone.”
However, the consensus in the field is that mood stabilization should be achieved first, before low to moderate (but not high) doses of stimulants are added. “Thus, in the face of an inadequate response to the lithium-risperidone combination in this child, stimulants could be deferred until better mood stabilization was achieved.”
Other Approaches to Mood Stabilization and Anxiety Reduction
“The anticonvulsant mood stabilizers (carbamazepine, lamotrigine, and valproate) each have considerable mood stabilizing and anti-anxiety effects, at least in adults with bipolar disorder. With inadequate mood stabilization of this patient on lithium and risperidone, we would consider the further addition of lamotrigine.
Lamotrigine appears particularly effective in adults with bipolar disorder who have a personal history and a family history of anxiety (as opposed to mood disorders), and it has positive open data in adolescents with bipolar depression and in a controlled study of maintenance (in teenagers 13–17, but not in preteens 10–12) (Findling et al. 2015). With better mood stabilization, anxiety symptoms usually diminish…, and we would pursue these strategies [instead of using] antidepressants for depression and anxiety in young children with bipolar disorder.”
“Carbamazepine appears to be more effective in adults with bipolar who have [no] family history of mood disorders,” unlike lithium, which seems to work better in people who do have a family history of mood disorders.
“While the overall results of oxcarbazepine in childhood mania were negative, they did exceed placebo in the youngest patients (aged 7–12) as opposed to the older adolescents (13–18) (Wagner et al. 2006).
“There are long-acting preparations of both carbamazepine (Equetro) and oxcarbazepine (Oxtellar) that would allow for all nighttime dosing to help with sleep and reduce daytime side effects and sedation. Although data [on] anti-manic and antidepressant effects in adults are stronger for carbamazepine than oxcarbazepine,” there are good reasons to consider oxcarbazepine. First, there is the finding mentioned above that oxcarbazepine worked best in the youngest children. Second, there is a lower incidence of severe white count suppression on oxcarbazepine. Third, it has less of an effect on liver enzymes than carbamazepine. However, low blood sodium levels are more frequent on oxcarbazepine than carbamazepine.
Other Atypical Antipsychotics That May Improve Depression
Management of Unipolar and Bipolar Depression During Pregnancy
 At the Maryland Psychiatric Research Society’s continuing medical education conference in November, Lauren Osbourne, Assistant Director of the Women’s Mood Disorders Clinic at Johns Hopkins Hospital, gave a presentation on the management of mood and anxiety during pregnancy and lactation. She had a number of important ideas for physicians and patients to consider in their decision-making process.
At the Maryland Psychiatric Research Society’s continuing medical education conference in November, Lauren Osbourne, Assistant Director of the Women’s Mood Disorders Clinic at Johns Hopkins Hospital, gave a presentation on the management of mood and anxiety during pregnancy and lactation. She had a number of important ideas for physicians and patients to consider in their decision-making process.
According to Osbourne, 60%-70% of pregnant women with unipolar depression who discontinue their antidepressants relapse. Of those with bipolar disorder who discontinue their mood stabilizers, 85% relapse, while 37% of those who stay on their medications relapse.
Something to consider when deciding whether to continue medication while pregnant is that depression in pregnancy carries its own risks for the fetus. These include preterm delivery, low birth weight, poor muscle tone, hypoactivity, increased cortisol, poor reflexes, and increased incidence of attention deficit hyperactivity disorder (ADHD) and other behavioral disorders.
The placenta makes an enzyme 11-BHSD2 that lowers the stress hormone cortisol in the baby. However, this enzyme is less active in depression, exposing the fetus to higher levels of cortisol.
Thus, the decision about whether to continue medications during pregnancy should consider the risks to the fetus of both the mother’s depression and the mother’s medications.
Most antidepressants are now considered safe during pregnancy. There have been reports of potential problems, but these data are often confounded by the fact that women with more severe depression are more likely to require antidepressants, along with other risk variables such as smoking or late delivery (after 42 weeks). When these are accounted for by using matched controls, the apparent risks of certain antidepressants are no longer significant. This includes no increased risk of persistent pulmonary hypertension, autism, or cardiac malformations.
There may be a possible increased risk of Neonatal Adaption Syndrome (NAS) in the first weeks of life in babies who were exposed to selective serotonin reuptake inhibitor (SSRI) antidepressants in the third trimester. This syndrome presumably results from antidepressant withdrawal, and can include respiratory distress, temperature changes, decreased feeding, jitteriness/irritability, floppiness or rigidity, hypoglycemia, and jaundice. There is not yet a robust literature on the syndrome, but Osbourne suggested that it disappears within 2 weeks of birth.
In her practice, Osbourne prefers to prescribe sertraline, which has the best safety data, along with fluoxetine. Sertraline is also OK for breastfeeding. There is less data on bupropion, but it also appears to be safe during pregnancy. Endocrine and enzyme changes in pregnancy typically cause a 40% to 50% decrease in concentrations of antidepressants, so doses of antidepressants typically must be increased in order to maintain their effectiveness.
Osbourne ranked mood stabilizers for bipolar disorder, from safest to most worrisome. Lamotrigine is safest. There is no evidence linking it to birth defects, but higher doses are required because of increased clearance during pregnancy. Lithium is next safest. There are cardiac risks for one in 1,200 patients, but these can be monitored. Carbamazepine is third safest. One percent of babies exposed to carbamazepine will develop spina bifida or craniofacial abnormalities. Valproate is least safe during pregnancy. Seven to ten percent of babies exposed to valproate will develop neural tube defects, other malformations, or developmental delay, with a mean decrease of 9 IQ points. The atypical antipsychotics all appear safe so far.
Alternatives and Adjuncts to Medications in Pregnancy
Simvastatin Looks Promising in Treatment of Negative Symptoms of Schizophrenia
The statin drug simvastatin (Zocor) enhances the effects of risperidone on negative symptoms of schizophrenia, according to a 2017 article by Soode Tajik-Esmaeeli and colleagues in the journal International Clinical Psychopharmacology.
In the 8-week study, 40 mg/day of simvastatin enhanced the effects of 4–6 mg/day of the antipsychotic risperidone on negative symptoms of schizophrenia, such as apathy and withdrawal, but not positive symptoms such as hallucinations or delusions.
Other statins, lovastatin and pravastatin, have not had a similar effect, possibly because they do not cross the blood-brain barrier as easily as simvastatin does.
Simvastatin has other benefits as well. Like all statins it decreases lipid levels, reducing cardiovascular disease. People with schizophrenia and bipolar disorder are at especially high risk for cardiovascular disease.
Simvastatin also decreases inflammation (lowering IL-1 alpha and TNF-beta levels) and may be neuroprotective, as it increases brain-derived neurotrophic factor (BDNF), a protein that protects neurons and is important for learning and memory. Inflammation is increasingly implicated in schizophrenia and bipolar disorder.
There is also some evidence that statins can prevent depressions over long-term follow-up. Studies in women without depression and men who had recently had heart attacks both showed that those taking statins had a lower rate of future depression than those not taking statins.
Editor’s Note: These findings suggest a potential 5-fold benefit to simvastatin: 1) It reduces negative symptoms in schizophrenia. 2) It reduces inflammation. 3) It increases BDNF. 4) It decreases cardiovascular disease risk by lowering lipid levels. 5) It may prevent future depressions.
Other approaches to augmenting schizophrenia treatment include nutritional supplements vitamin D3 and folate. Patients with psychosis often have vitamin D deficits. Folate supplements can reduce homocysteine, which has been linked to cognitive deficits in schizophrenia.
Probiotics May Improve Depression As Well As IBS
 A pilot study of people with irritable bowel syndrome (IBS) suggests that taking a probiotic nutritional supplement can improve depression as well as gastrointestinal upset.
A pilot study of people with irritable bowel syndrome (IBS) suggests that taking a probiotic nutritional supplement can improve depression as well as gastrointestinal upset.
In the 2017 study published in the journal Gastroenterology, researcher Maria Pinto Sanchez and colleagues at the Farncombe Family Digestive Health Research Institute found that when those with IBS took a probiotic, their co-occurring depression improved more than it did in people with IBS who took a placebo.
Senior author Premysl Bercik suggested the study confirms that the microbiota environment in the gut affects what goes on in the brain, opening new avenues for the treatment of psychiatric diseases.
The study included 44 adults with IBS who also had mild to moderate anxiety and depression. For 10 weeks, half received a daily dose of the probiotic Bifidobacterium longum NCC3001, while the others received placebo.
After 6 weeks, 64% of the probiotic group saw improvement in their depression, compared to 32% of the placebo group. Functional magnetic resonance imaging (fMRI) showed brain changes associated with the improvement in mood.
The researchers are planning larger trials of probiotics.