Lithium Extends Anti-Depressant Effects of Ketamine in Mice
While it can sometimes take weeks for the effects of antidepressant treatments to appear, intravenous ketamine can produce antidepressant effects in as little as two hours. However, ketamine’s effects fade after three to five days. New animal research by Chi-Tso Chiu et al. explores whether adding lithium to ketamine treatment can produce more sustained antidepressant effects.
Mice who are restrained by being placed in a tube for several hours (chronic restraint stress) exhibit a behavioral and neurochemical profile that resembles human depression. When Chiu and colleagues pretreated these stressed mice with sub-therapeutic doses of lithium (600 mg/L) in their drinking water for several weeks, a sub-therapeutic dose of ketamine (2.5 mg/kg of body weight) was enough to produce robust antidepressant effects in the mice, while neither drug alone was effective at these doses.
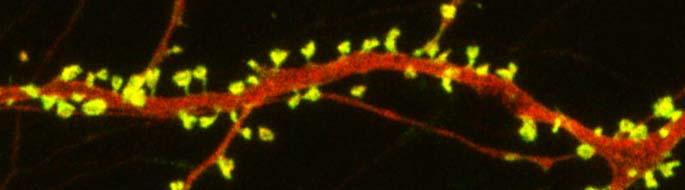
The combination of ketamine and lithium also restored the density of spines on the dendrites of neurons in the medial prefrontal cortex. Post-treatment with lithium (1200 mg/L) for several weeks was also successful in extending the effects of a single (50 mg/kg) ketamine injection.
Both lithium and ketamine affect the intracellular signaling pathway mTOR. Ketamine activates the pathway, increasing levels of synaptic proteins and dendritic spine density. It also increases brain-derived neurotrophic factor (BDNF) and the BDNF receptor TrkB. BDNF is important for learning and memory.
When lithium was added to the treatment of the mice with ketamine, the mTOR and BNDF pathways were further activated. Lithium also inhibits the receptor GSK-3, supporting ketamine’s rapid-acting antidepressant effects.
Ketamine treatment can produce oxidative stress, in which toxic free radicals can endanger cells, and the addition of low doses of lithium also completely prevented this neurochemical side effect.
Chiu and colleagues hope that the findings of this study in mice can eventually be applied to research in humans in the hopes of finding a clinical option that would sustain the rapid-onset antidepressant effects of ketamine for the long term.
In Mice, Autism-Like Behavior Connected to Problems Pruning Dendritic Spines
Autism spectrum disorders are associated with developmental abnormalities at excitatory synapses. Dendrites, the branched projections of neurons where electrical signals are passed from one cell to the next, are covered in hundreds to thousands of spines that facilitate the synaptic connections with other neurons. These spines are created and also pruned as part of normal learning and development.
Post-mortem examination of the brains of patients with autism spectrum disorders shows increased density of dendritic spines and less pruning in certain neurons in the temporal lobe. These examinations also show impaired mTOR autophagy. MTOR is a protein that plays a role in cell growth and survival. Autophagy is the normal process by which some components of cells are broken down.
A 2014 study by Guomei Tang et al. in the journal Neuron showed that mice that are genetically altered to have overactive mTOR also have reduced dendritic spine pruning, blockade of autophagy, and increased autism-like behaviors. An immunosuppressant drug called rapamycin inhibits mTOR, and treating the mice with this drug corrected the problems with spine pruning and the autism-like behaviors. (This was not true for mice who had been altered to have another type of autophagy.) Normal spine formation was not affected by the restored pruning ability.
Tang et al. concluded that mTOR autophagy plays an important role in dendritic spine pruning, and that restoring neuronal autophagy can correct synaptic abnormalities and restore normative social behavior in mice with hyperactive mTOR.