Astrocytes Can Turn Toxic
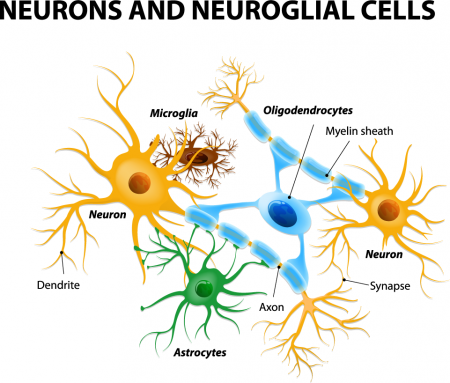
Astrocytes usually play a helpful role in the brain. These glial cells facilitate neural connections and prune unnecessary ones. However, new research details how infection or trauma can render astrocytes toxic, leading to brain disorders. In a 2017 article in the journal Nature, researcher Shane A. Liddelow and colleagues describe how resting astrocytes can become harmful reactive astrocytes.
The researchers determined that reactive astrocytes are found in brain tissues following brain injuries, or in neurological disorders including Alzheimer’s, Parkinson’s, Huntington’s, amyotrophic lateral sclerosis (ALS), and multiple sclerosis. Liddelow and colleagues also determined that microglia play a role in transforming resting astrocytes into a subtype of harmful reactive astrocytes, dubbed A1 astrocytes, and that the latter secrete a neuron-killing toxin.
A1 astrocytes lose their ability to promote neuronal survival and to create new synapses. The researchers showed that blocking the formation of A1 astrocytes prevented certain neuron deaths, and they hope this finding will lead to new treatments for brain injuries and neurological disorders.
Editor’s Note: It is possible that A1 astrocytes are also induced in patients with bipolar disorder, post-traumatic stress disorder (PTSD), and schizophrenia, as well as head traumas and neurological disorders.
Agomelatine in an Animal Model of PTSD
At the 2014 meeting of the International College of Neuropsychopharmacology, researcher Joseph Zohar presented a poster on the effects of early post-stressor intervention with the drug agomelatine in animals who showed behavioral and molecular responses to stress that served as a model of post-traumatic stress disorder (PTSD).
Agomelatine is available clinically as an antidepressant in Canada and Europe (but not in the US), and can also reduce anxiety and re-synchronize circadian rhythms. Agomelatine is a melatonin (MT1/MT2) receptor agonist and a serotonin 5HT2C antagonist (increasing dopamine and norepinephrine in the frontal cortex).
Long-term behavioral, molecular and structural effects of the drug were assessed in animals. Adult male Sprague-Dawley rats were exposed to the scent of a predator for 10 minutes, and one hour later they were treated acutely for this stress with agomelatine (50mg/kg i.p.) or placebo.
Agomelatine decreased the prevalence of extreme, PTSD-like behavioral and molecular responses to the stressor, such as freezing in place and increased corticosterone. Agomelatine also normalized decreases in brain-derived neurotrophic factor (BDNF) observed in the dentate gyrus of the hippocampus, the cortex (layer III), and the basolateral amygdala. In line with this, agomelatine-treated stressed animals displayed significantly increased number and length of dendrites at glutamate synapses in the hippocampus (including the dentate gyrus and CA1) and reversed the hippocampal neuronal retraction observed in the rats who were given the placebo.
Agomelatine also affected the expression of clock genes in the rats, which regulate biorhythms. These genes lead to the production of the major clock gene proteins Per1 and Per2. Agomelatine normalized Per1 increases in three parts of the brain: the CA3, another glutamate synapse near the dentate gyrus; the suprachiasmatic nucleus over the optic chiasm, important for circadian rhythms; and the basolateral amygdala. Per2, a protein that also drives circadian rhythms, increased in the CA1 synapse of the hippocampus, the suprachiasmatic nucleus and the basolateral amygdala of the stressed rats.
The researchers concluded that the data provide “initial evidence that a single dose of agomelatine administered in the acute aftermath of stress promotes recovery while promoting enhanced neuronal and synaptic plasticity and connectivity in the secondary prevention of PTSD in this model.”
In Mice, Autism-Like Behavior Connected to Problems Pruning Dendritic Spines
Autism spectrum disorders are associated with developmental abnormalities at excitatory synapses. Dendrites, the branched projections of neurons where electrical signals are passed from one cell to the next, are covered in hundreds to thousands of spines that facilitate the synaptic connections with other neurons. These spines are created and also pruned as part of normal learning and development.
Post-mortem examination of the brains of patients with autism spectrum disorders shows increased density of dendritic spines and less pruning in certain neurons in the temporal lobe. These examinations also show impaired mTOR autophagy. MTOR is a protein that plays a role in cell growth and survival. Autophagy is the normal process by which some components of cells are broken down.
A 2014 study by Guomei Tang et al. in the journal Neuron showed that mice that are genetically altered to have overactive mTOR also have reduced dendritic spine pruning, blockade of autophagy, and increased autism-like behaviors. An immunosuppressant drug called rapamycin inhibits mTOR, and treating the mice with this drug corrected the problems with spine pruning and the autism-like behaviors. (This was not true for mice who had been altered to have another type of autophagy.) Normal spine formation was not affected by the restored pruning ability.
Tang et al. concluded that mTOR autophagy plays an important role in dendritic spine pruning, and that restoring neuronal autophagy can correct synaptic abnormalities and restore normative social behavior in mice with hyperactive mTOR.
Thalamus Implicated in Depression-Like Behavior and Resilience to It
At the 2014 meeting of the International College of Neuropsychopharmacology, researcher Scott Russo described characteristics of rodents who showed depression-like behavior after 10 days of exposure to a larger, more aggressive animal (a phenomenon known as defeat stress). These animals exhibited many behaviors that resembled human depression, including anxiety-like behaviors while navigating a maze; activation of the hypothalamic-pituitary-adrenal axis; circadian rhythm abnormalities; metabolic changes such as glucose intolerance; susceptibility to addiction; anhedonia, a lack of interest in sucrose, sex or intracranial self-stimulation; and profound and permanent social avoidance.
In susceptible animals, Russo found anatomical changes in the GABAergic neurons of the nucleus accumbens (also known as the ventral striatum), including increased numbers of synapses and a greater number of stubby spines on dendrites (the branched projections of neurons where electrical signals are passed from one cell to the next), as well as greater excitability of glutamatergic input, observed as excitatory post-synaptic potentials.
Russo’s attempt to identify these key neurons among the billions of neurons and the 100 to 500 trillion synapses in the brain was like the search for a needle in a haystack, but thinks he found it. The medium spiny neurons of the nucleus accumbens contain GABA and receive synapses from the prefrontal cortex, amygdala, and intralaminar nucleus of the thalamus (ILT), in addition to dopamine inputs from the VTA, and cholinergic, somatostatin, and orexin inputs. Russo found that it was the ILT inputs that conveyed susceptibility to defeat stress, and their presynaptic endings showed increased levels of glutamate transporters (VGLUT-2). Driving the ILT was sufficient to cause the rodents to display the depression-like behaviors, and silencing the ILT during defeat stress prevented the susceptible behaviors (like social avoidance) and promoted resilience.
Glia Cells Prune Over-Abundant Neurons
The brain contains neurons, which transmit electrical impulses, and glia, which protect and support neurons. New evidence suggests that some types of glia also play a role in pruning back overabundant neurons that are produced as the brain develops in utero.
Researcher Beth Stevens reports that astrocytes secrete a protein called transforming growth factor beta (TGF-beta). TGF-beta is a cytokine, or regulating protein, that activates brain microglia to initiate a complement cascade (C1 to C3), a series of chemical changes that destroy unnecessary neurons and synapses.
The various proteins involved in a complement cascade are numbered. This complement cascade starts with C1q and is continued by C4, C2, and C3, which initiate phagocytosis (or eating up) of the axon terminals of the underutilized neurons, sparing those that are active.
Inflammation and other changes in glia could cause either deficient or excess pruning of neurons, which has been thought to occur in neuropsychiatric disorders such as autism or schizophrenia.