Over-Pruning of Synapses May Explain Schizophrenia
A gene that plays a role in the pruning of synapses has been linked to schizophrenia. The gene encodes an immune protein called complement component 4 (C4), which may mediate the pruning of synapses, the connections between neurons. Researchers led by Aswin Sekar found that in mice, C4 was responsible for the elimination of synapses. The team linked gene variants that lead to more production of C4A proteins to excessive pruning of synapses during adolescence, the period during which schizophrenia symptoms typically appear. This may explain why the brains of people with schizophrenia have fewer neural connections. The researchers hope that future therapies may target the genetic roots of the illness rather than simply treating its symptoms.
Memory Activates Epigenetic Changes in Mice Brain Cells
In a 2015 article in Nature Neuroscience, Stefan Bonn and André Fischer reported that when mice were prompted to use their long-term memory to recognize a specific environment, epigenetic changes occurred in their neurons and glia. Epigenetic changes refer to chemical alterations in DNA or histones (which give DNA structure) that increase or decrease the expression of certain genes. Sometimes environmental factors lead to a methyl or acetyl group joining a strand of DNA or histones, changing how easily the genes are turned on or off.
When the mice used their long-term memory, the main change that occurred was DNA methylation in their neurons. There were also changes to histones that were linked to memory acquisition but resulted in few changes in gene expression. The DNA methylation changes, on the other hand, changed neural pathways, leading to “rewiring” of the brain.
Brain Inflammation in People at High Risk for Schizophrenia
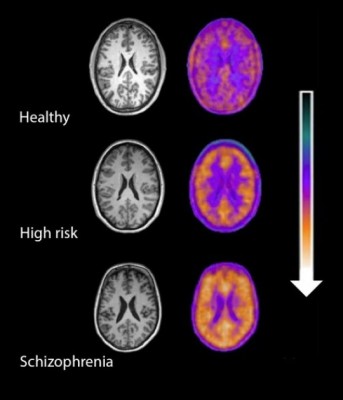
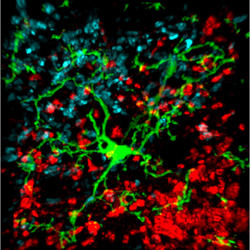
Microglial activity in the brains of people who are healthy, people at high risk for schizophrenia, and people who have been diagnosed with schizophrenia.
A 2016 study by Peter S. Bloomfield and colleagues in the American Journal of Psychiatry used PET scans to compare the activity of microglia, immune cells in the central nervous system, in healthy controls, people with schizophrenia, and those at high risk for the illness. It found that both people with schizophrenia and those at high risk had greater brain inflammation than the healthy controls.
The study was the first to show that microglial activity was elevated in people at high risk (who showed some preliminary symptoms of schizophrenia). The finding had a large effect size.
Microglial activity was also correlated with symptom severity in the high-risk participants. Increased microglial activity was not linked to depression, suggesting that it is specific to the development of psychosis.
These findings resemble those of other recent studies showing increased inflammation in people at high risk for psychosis.
The study suggests that increased microglial activity occurs before a first episode of psychosis. That means it could help identify people who may develop schizophrenia. The findings also suggest that anti-inflammatory treatment could theoretically be used to prevent psychosis.
RTMS for Depression Increases Volume of Specific Brain Regions
Repeated transcranial magnetic stimulation (rTMS) is a treatment for depression in which magnets placed near the skull stimulate electrical impulses in the brain. In a poster presented at the 2015 meeting of the Society of Biological Psychiatry, Martin Lan and colleagues presented results of the first study of structural changes in the brain following rTMS.
In the study, 27 patients in an episode of major depression underwent magnetic resonance brain scans before and after receiving rTMS treatment over their left prefrontal cortices. Lan and colleagues reported that several cortical regions related to cognitive appraisal, the subjective experience of emotion, and self-referential processing increased in volume following rTMS treatment: the anterior cingulate, the cingulate body, the precuneous, right insula, and gray matter in the medial frontal gyrus. The increases ranged from 5.3% to 15.7%, and no regions decreased in volume. More than 92% of the participants showed increased gray matter in all of these regions.
The brain changes were not correlated with antidepressant response to rTMS, but suggest a possible mechanism by which rTMS is effective in some people. Lan and colleagues concluded that rTMS likely had neuroplastic effects in areas of the brain that are important for emotion regulation.
Primates Shed Light on the Neurobiology of Anxiety Disorders in Children
Studies of primates suggest that the amygdala plays an important role in the development of anxiety disorders. Researcher Ned Kalin suggested at the 2015 meeting of the Society of Biological Psychiatry that the pathology of anxiety begins early in life. When a child with anxiety faces uncertainty, the brain increases activity in the amygdala, the insula, and the prefrontal cortex. Children with an anxious temperament, who are sensitive to new social experiences, are at almost sevenfold risk of developing a social anxiety disorder, and later experiencing depression or substance abuse.
A study by Patrick H. Roseboom and colleagues presented at the meeting was based on the finding that corticotropin-releasing hormone (CRH) plays a role in stress and is found in the central nucleus of the amygdala (as well as in the hypothalamus). The researchers used viral vectors to increase CRH in the central nucleus of the amygdala in young rhesus monkeys, hoping to determine what impact increased CRH has on a young brain. Rhesus monkeys and humans share similar genetic and neural structures that allow for complex social and emotional functioning.
Roseboom and colleagues compared the temperaments of five monkeys who received injections increasing the CRH in their amygdala region to five monkeys who received control injections. As expected, the monkeys with increased CRH showed increases in anxious temperament. Brain scans also revealed increases in metabolism not only in the central nucleus of the amygdala, but also in other parts of the brain that have been linked to anxiety, including the orbitofrontal cortex, the hippocampus, and the brainstem, in the affected monkeys. The degree of increase in amygdala metabolism was directly proportional to the increase in anxious temperament in the monkeys, further linking CRH’s effects in the amygdala to anxiety.
Adolescence is a Sensitive Period for Fear Learning
Adolescence can be a time of vulnerability to illness. Anxiety disorders increase during this period, and three-quarters of adults with anxiety disorders trace the illness back to their childhood or adolescence. The most common treatments for anxiety disorder are based on the idea of fear extinction. A certain stimulus, like a social situation or seeing a spider, provokes a fear reaction in the brain. Through gradually increasing exposure to the stimulus and extinction training, the person becomes desensitized to the stimulus. New research on rodents presented by Francis S. Lee at the 2015 meeting of the Society for Biological Psychiatry suggests that the extinction process is diminished during adolescence.
At specific stages of maturation, neural circuits related to particular abilities can become flexible. Brain and behavior become sensitive to and are increasingly shaped by experience. Studies of rodents and humans have shown that adolescence is a time when the neural circuitry for fear extinction is in flux. In mice, this period falls around their 29th day of life. Lee reported that around this time, the mice begin to exhibit resistance to extinction of fear learning.
In adolescent rodents, there is a surge of contextual fear learning and retrieval that is mediated by hyper-connectivity of the ventral hippocampus and the amygdala to the prelimbic part of the prefrontal cortex. In contrast, the pathway from the amygdala to the infralimbic cortex mediates the extinction of this type of learning. Because the prelimbic pathway for fear learning is overactive, the infralimbic pathway for extinction learning is less effective.
Adolescent mice temporarily lose their ability to retrieve memories related to cue-dependent (as opposed to context-dependent) fear learning. Remarkably, when these animals proceed into adulthood, the fear learning associated with cues returns and becomes accessible again.
This could help explain how teenagers can lose fear conditioning to cues (for example, speeding through a red light) they learned in childhood. The fear is forgotten (or becomes inaccessible) in adolescence, but then what had been learned is again “remembered” (retrieved) in adulthood. Read more
Molecular Biology of Depression
 Dysregulation of the brain in early life can have lasting effects, and the effects of stress and depression can also accumulate. At the 2015 meeting of the Society of Biological Psychiatry, researcher Huda Akil explained that behavioral pathology can “take on a life of its own, leading to deteriorating course of illness and treatment resistance.” She illustrated how preclinical work in animals can help clarify the molecular biology of depression and develop new targets for therapeutics.
Dysregulation of the brain in early life can have lasting effects, and the effects of stress and depression can also accumulate. At the 2015 meeting of the Society of Biological Psychiatry, researcher Huda Akil explained that behavioral pathology can “take on a life of its own, leading to deteriorating course of illness and treatment resistance.” She illustrated how preclinical work in animals can help clarify the molecular biology of depression and develop new targets for therapeutics.
Early Life Experiences are Key
Akil discuss studies of rodents in which she used new molecular genetic techniques to increase the number of glucocorticoid receptors in the hippocampus early in life (prior to weaning). Glucocorticoid receptors mediate the effects of the stress hormone cortisol in people and corticosterone in rodents. More receptors help shut off cortisol secretion after a stressful event. People with post-traumatic stress disorder (PTSD) have high levels of glucocorticoid receptors while people with depression have low levels, leading to over-secretion of cortisol in depression.
The increased glucocorticoid receptors led to a long-term increase in anxiety behaviors and response to stimulants. When Akil carried out the same manipulation on rats that had already been weaned, it had no long-lasting effects, showing that there is a vulnerability window for some long-lasting effects on behavior.
CLOCK Genes and Circadian Rhythms
Akil also studied CLOCK genes in rodents. These genes, including BMAL-1, Per 1, Per 2, and Per3, play a role in circadian rhythms, and their transcription induces these 24-hour cycles. In rodents who were induced into a depression-like state, the CLOCK genes were dysregulated and did not correspond to normal circadian rhythms. These data show that depressive states can induce changes in CLOCK genes and circadian rhythms. Others have shown the converse, that abnormal CLOCK genes can induce behavioral abnormalities including mania-like behaviors.
Fibroblast Growth Factor
Levels of fibroblast growth factor 2 (FGF2) in the hippocampus are low in people with depression. In rodents, FGF2 inhibits anxiety. Decreases in FGF2 are seen in the hippocampus of animals in a depression-like state following repeated defeat by a larger animal. It appears that FGF2 is an endogenous antidepressant (i.e. one that is produced by the brain). When the rodent brain is manipulated to eliminate FGF2, the animals become anxious.
In addition, animals bred to have high stress, low social responsivity, and resistance to new learning also have low FGF2. Treatment with FGF2 reversed these behavioral abnormalities and also increased the production of new neurons. For the stressed rats, receiving FGF2 on their second day of life increased new neuron production, decreased anxiety, decreased proneness to social defeat stress and increased the bonding hormone oxytocin in the amygdala into adulthood.
FGF2 had no effect on rats bred for low stress and high social responsivity, indicating that it only worked for the rats that needed it. Akil compared FGF2 to “personalized medicine for rats.”
Defeat stress affects the way genes are transcribed, and FGF2 was able to reverse one of these specific transcriptional effects, suggesting it could potentially ameliorate some of the long-lasting effects of stress and depression.
The Human Brain
Akil also studied the brains of people who had died of depression, bipolar disorder, or schizophrenia. In bipolar disorder, the nucleus accumbens, the reward center of the brain, was enlarged.
In contrast, Akil described the brains of those people who had died with depression as being “low on fertilizer.” That is, they showed less cell growth, less production of new neurons, more abnormalities in cell shape, and more cell death. Akil said that by the time someone is severely ill, the pathology is all over the brain. The changes Akil saw in the brains of people who were depressed are also consistent with data indicating that several neuroprotective factors, including BDNF and VEG-F, are low in the frontal cortex and the hippocampus of depressed people (while BDNF is high in the nucleus accumbens).
Blood and Now Brain Inflammation Linked to Depression
 There is growing evidence of a link between inflammation of depression. At the 2015 meeting of the Society of Biological Psychiatry, researcher Jeff Meyer summarized past studies on inflammatory markers. These are measurements, for example of certain proteins in the blood, that indicate the presence of inflammation in the body.
There is growing evidence of a link between inflammation of depression. At the 2015 meeting of the Society of Biological Psychiatry, researcher Jeff Meyer summarized past studies on inflammatory markers. These are measurements, for example of certain proteins in the blood, that indicate the presence of inflammation in the body.
Common inflammatory markers that have been linked to depression include IL-6, TNF-alpha, and c-reactive protein. At the meeting, Meyer reviewed the findings on each of these. Twelve studies showed that IL-6 levels are elevated in the blood of patients with depression. Four studies had non-significant results of link between IL-6 and depression, and Meyer found no studies indicating that IL-6 levels were lower in those with depression. Similarly, for TNF-alpha, Meyer found 11 studies linking elevated TNF-alpha with depression, four with non-significant results, and none showing a negative relationship between TNF-alpha and depression. For c-reactive protein, six studies showed that c-reactive protein was elevated in people with depression, six had non-significant results, and none indicated that c-reactive protein was lower in depressed patients.
Most studies that have linked inflammation to depression have done so by measuring inflammatory markers in the blood. It is more difficult to measure inflammation in the brain of living people, but Meyer has taken advantage of new developments in positron emission tomography (PET) scans to measure translocator protein binding, which illustrates when microglia are activated. Microglial activation is a sign of inflammation. Translocator protein binding was elevated by about 30% in the prefrontal cortex, anterior cingulate cortex, and insula in study participants who showed symptoms of a major depressive episode compared to healthy control participants. The implication is that the depressed people with elevated translocator protein binding have more brain inflammation, probably via microglial activation.
The antibiotic minocycline reduces microglial activation. It would be interesting to see if minocycline might have antidepressant effects in people with depression symptoms and elevated translocator protein binding.
Autopsy Studies Show Brain Inflammation in Unipolar Depression, Bipolar Disorder, and Suicide
 Depression and bipolar disorder have been linked to high levels of inflammatory proteins in the blood (namely CRP, IL-1, IL-6, and TNF-alpha), but the relationship between these illnesses and inflammation in the brain has not been well-characterized.
Depression and bipolar disorder have been linked to high levels of inflammatory proteins in the blood (namely CRP, IL-1, IL-6, and TNF-alpha), but the relationship between these illnesses and inflammation in the brain has not been well-characterized.
At the 2015 meeting of the Society for Biological Psychiatry, researcher Ghanshyan Pandey discussed findings from autopsy studies of people who died with a diagnosis of unipolar depression or bipolar disorder, and teens who died of suicide. The studies compare data from these ill people with those of controls who are matched for demographic characteristics.
Pandey found that the brains of those who died of unipolar depression and bipolar disorder showed more signs of inflammation compared to the controls. This included elevated levels of the inflammatory proteins IL-1B, IL-6, and TNF-alpha, in addition to elevated levels of the mRNA that leads to their production. Pandey also found that those with depression and bipolar disorder had higher levels of mRNA for the receptors to which TNF-alpha and other inflammatory proteins attach themselves.
Pandey performed similar autopsy studies of teens who died of suicide, the second leading cause of death for this age group, compared to teens who died of other causes. There were more signs of inflammation in the prefrontal cortices of teens who died of suicide. These included mRNA and proteins for IL-1B and TNF-alpha, and IL-6 proteins. In contrast to the ill adults, the teens who died of suicide had lower levels of the receptors for inflammatory proteins than controls. Another type of receptor known as toll-like receptors was higher in the ill teens, particularly the mRNA and proteins TLR3 and TLR4.
Chronic Drug Use and Recovery
 George Koob, Director of the National Institute on Alcohol Abuse and Alcoholism, discussed the neuroscience of chronic drug use at the 2015 meeting of the Society of Biological Psychiatry. His basic message was that chronic drug use is associated with A) loss of the reward value of the drug and B) a progressive increase in dysphoria and stress when off the drug. Both factors drive craving and drug seeking.
George Koob, Director of the National Institute on Alcohol Abuse and Alcoholism, discussed the neuroscience of chronic drug use at the 2015 meeting of the Society of Biological Psychiatry. His basic message was that chronic drug use is associated with A) loss of the reward value of the drug and B) a progressive increase in dysphoria and stress when off the drug. Both factors drive craving and drug seeking.
Access to high as opposed to moderate doses of a drug lead to an escalation in drug intake, and associated persistent increases in withdrawal dysphoria, which Koob called “the dark side.”
Koob explained that a month of detoxification is not sufficient, and that people quitting a drug need more time to let dopamine increase and to let levels of corticotropin releasing factor (CRF), which drives the anxiety and dysphoria of withdrawal, normalize. He stressed that for people addicted to opiates, it is important to taper levels of the drug to minimize withdrawal symptoms.
In addition to CRF, dynorphin also plays a role in chronic drug abuse. This opiate peptide acts at kappa opiate receptors and is associated with anxiety, dysphoria, and psychosis as opposed to morphine, which acts at mu opiate receptors and is associated with euphoria and decreased pain. Koob found that administration of the kappa opiate antagonist norbinaltorphimine (nor-BNI) blocks dose escalation of methamphetamine and brings abstinence-related compulsive drug seeking back to baseline.