New Data on Vortioxetine for Cognition in Unipolar Depression
A 5mg dose of the antidepressant vortioxetine (Brintellix) was previously reported to have positive cognitive effects in elderly depressed patients. In a 2014 article in the International Journal of Neuropsychopharmacology, researcher Roger S. McIntyre et al. presented data from FOCUS, a study of cognition in depressed patients. The eight-week double-blind study included 18- to 65-year-olds (who were not selected for having cognitive problems per se).
McIntyre and colleagues used two tests of cognition, the Digit Symbol Substitution Test (DSST), which measures attention, psychomotor speed, and executive function, and the Rey Auditory Verbal Learning Test (RAVLT), which measures memory and acute and delayed recall. The researchers found that both the 195 patients taking 10mg/day of vortioxetine and the 207 patients taking 20mg/day of vortioxetine had better performance on both tests than the 196 patients who received placebo.
Response rates (meaning a patient achieved a 50% improvement on a scale of depression) were 47.7% on 10mg of vortioxetine, and 58.8% on 20mg of vortioxetine, compared to 29.4% on placebo. Remission rates were 29.5% on 10mg of vortioxetine and 38.2% on 20mg of vortioxetine versus 17% on placebo. McIntyre suggested that the drug worked both directly and indirectly, improving depression in some, but also improving cognition even in those whose depression did not improve.
The mechanism that could account for vortioxetine’s cognitive effects has not yet been identified. Like other selective serotonin reuptake inhibitor (SSRI) antidepressants, vortioxetine is a potent blocker of serotonin (5HT) reuptake, which it does by inhibiting the serotonin transporter (5HT-T). Unlike other SSRIs, vortioxetine is also a blocker of 5HT3 and 5HT7 receptors, an agonist at 5HT1A and 5HT1B and a partial agonist at 5HT1D receptors. It could be considered a polymodal 5HT active drug in contrast to the more selectively active 5HT-T–inhibiting SSRIs.
Lighting Up Neural Networks in Mice
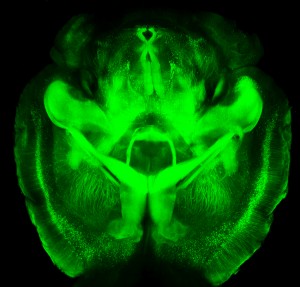 A new technology is making it possible to view the mammalian brain’s structure and connectivity for the first time. Karl Deisseroth discussed the technology, called CLARITY, at a plenary lecture at the 2014 meeting of the International College of Neuropsychopharmacology.
A new technology is making it possible to view the mammalian brain’s structure and connectivity for the first time. Karl Deisseroth discussed the technology, called CLARITY, at a plenary lecture at the 2014 meeting of the International College of Neuropsychopharmacology.
The way CLARITY works is by replacing lipids in the brain with a hydrogel substance. This preserves the structure of the brain’s neural networks, leaves proteins and nucleic acids intact, but allows for observation by rendering the brain transparent. This can be done in a system as large as the entire adult mouse brain. Early attempts took a whole day, but Deisseroth eventually found a way to render a mouse’s brain transparent in a matter of minutes.
The pictures are truly amazing, allowing for the visualization of previously microscropic neurons, dendrites, axons and connections in life-sized images. Pictures and details are available at www.clarityresourcecenter.org.
Deisseroth and colleagues have used CLARITY imaging to determine where neurons fire during different social activities. By placing photosensitive fibers in selected neurons using a virally based gene insertion technique, Deisseroth and colleagues were able to selectively fire dopamine neurons in the ventral tegmental area, part of the brain’s reward system, and thus increase or decrease the social interaction of mice by increasing or decreasing firing. The effects were selective to social interaction; the firing did not affect locomotor activity or exploration of an inanimate object.
The ventral tegmental area contains neurons that project to several locations in the brain, and Deisseroth and colleagues hoped to observe which were important to social interaction. Stimulating the ventral tegmental area to drive the medial prefrontal cortex caused anxiety in the mice and made them averse to social interaction. However, when the ventral tegmental area was used to selectively drive the nucleus accumbens, another part of the brain’s reward system, social interaction increased.
Deisseroth wanted to know if the nucleus accumbens was also involved in normal spontaneous social interactions. The researchers used a virus to insert an opsin-sensitive calcium gene that could give an ongoing readout of neural activity. (Opsin is a light-sensitive receptor found in cells in the retina.) The team found that the nucleus accumbens was implicated in social interaction with another mouse, but not in exploration of a novel object. Based on CLARITY imaging of the structure of ion channels (which are so small they cannot even be seen with an electron microscope), Deisseroth was able to selectively alter ion fluxes and turn neuronal firing on or off at will.
In the last 50 years, the brain and its billions of neurons and hundreds of trillions of synapses have gone from complete inaccessibility toward increasing clarity.
Rapid-Onset Antidepressant Treatments
At the International College of Neuropsychopharmacology (CINP) World Congress of Neuropsychopharmacology in 2014, several presentations and posters discussed treatments that bring about rapid-onset antidepressant effects, including ketamine, isoflurane, sleep deprivation, and scopolamine.
Ketamine’s Effects
Multiple studies, now including more than 23 according to researcher William “Biff” Bunney, continue to show the rapid-onset antidepressant efficacy of intravenous ketamine, usually at doses of 0.5 mg/kg over 40 minutes. Response rates are usually in the range of 50–70%, and effects are seen within two hours and last several days to one week. Even more remarkable are the six studies (two double-blind) reporting rapid onset of antisuicidal effects, often within 40 minutes and lasting a week or more. These have used the same doses or lower doses of 0.1 to 0.2mg/kg over a shorter time period.
Attempts to sustain the initial antidepressant effects include repeated ketamine infusions every other day up to a total of six infusions, a regimen in which typically there is no loss of effectiveness. Researcher Ronald Duman is running a trial of co-treatment with ketamine and lithium, since both drugs block the effects of GSK-3, a kinase enzyme that regulates an array of cellular functions, and in animals the two drugs show additive antidepressant effects. In addition, lithium has been shown to extend the acute antidepressant effects of one night of sleep deprivation, which are otherwise reversed by a night of recovery sleep.
Ketamine’s effects are related to the neurotransmitter glutamate, for which there are several types of receptors, including NMDA and AMPA. Ketamine causes a large burst of glutamate presumably because it blocks NMDA glutamate receptors on inhibitory interneurons that use the neurotransmitter GABA, causing glutamatergic cells to lose their inhibitory input and fire faster. While ketamine blocks the effects of this glutamate release at NMDA receptors, actions at AMPA receptors are not blocked, and AMPA activity actually increases. This increases brain-derived neurotrophic factor (BDNF), which is also required for the antidepressant effects of ketamine. Ketamine also increases the effects of mTOR, a kinase enzyme that regulates cell growth and survival, and if these are blocked with the antibiotic rapamycin, antidepressant effects do not occur.
In animal studies, ketamine increases dendritic spine growth and rapidly reverses the effects of chronic mild unpredictable stressors on the spines (restoring their mature mushroom shape and increasing their numbers), effects that occur within two hours in association with its rapid effects on behaviors that resemble human depression.
About 50–70% of treatment-resistant depressed patients respond to ketamine. However, about one-third of the population has a common genetic variation of BDNF in which one or both valine amino acids that make up the typical val-66-val allele are replaced with methionine (producing val-66-met proBDNF or met-66-met proBDNF). The methionine variations result in the BDNF being transported less easily within the cell. Patients with these poorly functioning alleles of BDNF are less likely to get good antidepressant effects from treatment with ketamine.
Ketamine in Animal Studies
Researcher Pierre Blier reviewed the effects of ketamine on the neurotransmitters serotonin, norepinephrine, and dopamine. In rodents, a swim stress test is used to measure depression-like behavior. Researchers record how quickly the rodents give up trying to get out of water and begin to float instead. Blier found that ketamine’s effects on swim stress were dependent on all three neurotransmitters. For dopamine, ketamine’s effects were dependent on increases in the number of dopamine cells firing, not on the firing rate, and for norepinephrine, ketamine’s effects were dependent on increases in burst firing patterns. Each of these effects was dependent on glutamate activity at AMPA receptors. Given these effects, Blier believes that using ketamine as an adjunct to conventional antidepressants that tend to increase these neurotransmitters may add to its clinical effectiveness.
Important Anecdotal Clinical Notes
Blier reported having given about 300 ketamine infusions to 25 patients, finding that two-thirds of these patients responded, including one-third who recovered completely, while one-third did not respond to the treatment. Patients received an average of 12 infusions, not on a set schedule, but according to when they began to lose response to the last ketamine infusion. If a patient had only a partial response, Blier gave the next ketamine treatment at a faster rate of infusion and was able to achieve a better response. These clinical observations are among the first to show that more than six ketamine infusions may be effective and well tolerated. Read more



