Antidepressants Work Better in Major Depressive Disorder than Previously Thought
As we’ve written before, the popular media has sometimes questioned the efficacy of antidepressants for unipolar depression. A reanalysis of data from previous controlled trials of fluoxetine and venlafaxine that was recently published in the Archives of General Psychiatry provides new evidence that these drugs are significantly more efficacious than placebo in youth, adult, and geriatric populations with major depressive disorder.
The researchers concluded,
To our knowledge, this is the first research synthesis in this area to use complete longitudinal person-level data from a large set of published and unpublished studies. The results do not support previous findings that antidepressants show little benefit except for severe depression. The antidepressants fluoxetine and venlafaxine are efficacious for major depressive disorder in all age groups, although more so in youths and adults compared with geriatric patients. Baseline severity was not significantly related to degree of treatment advantage over placebo.
Study Calls Into Question FDA Black Box Warning about Suicide Risk in Youth Taking Antidepressants
In 2004, the Federal Drug Administration issued a “black box warning” about increased risk of suicidal thoughts and behavior in children and adolescents taking selective serotonin reuptake inhibitors (SSRIs). See here for an overview from the National Institute of Mental Health.
An article published in the Archives of General Psychiatry earlier this year analyzed data from studies of fluoxetine and venlafaxine in youth, adults, and geriatric patients to determine if antidepressant use is linked to suicide. The drugs decreased both depressive symptoms and suicidal thoughts and behavior in adults and the geriatric population. They seemed to have no effect on suicidal thoughts or behavior in the youth.
For youths, no significant effects of treatment on suicidal thoughts and behavior were found, although depression responded to treatment. No evidence of increased suicide risk was observed in youths receiving active medication.
Weight Gain and Metabolic Risks of Antipsychotic Drugs in Children and Adolescents
 A study published in the Journal of Child and Adolescent Psychopharmacology in late 2011 reviewed the various weight and metabolic side effects of drugs like olanzapine, clozapine, risperidone, quetiapine, and aripiprazole.
A study published in the Journal of Child and Adolescent Psychopharmacology in late 2011 reviewed the various weight and metabolic side effects of drugs like olanzapine, clozapine, risperidone, quetiapine, and aripiprazole.
Across 34 published head-to-head and placebo-controlled studies in youth with psychotic and bipolar disorders, weight gain ranged from 3.8 to 16.2 kg with olanzapine (n=353), 0.9-9.5 kg with clozapine (n=97), 1.9-7.2 kg with risperidone (n=571), 2.3-6.1 kg with quetiapine (n=133), and 0-4.4 kg with aripiprazole (n=451).
Parental Nurturing Linked to Greater Hippocampal Volume in Young Children
An article published by Medscape reports that in a recent study by Dr. Joan Luby of Washington University School of Medicine in St. Louis, non-depressed preschool children whose parents showed more nurturing behaviors during a mildly stressful task were found to have hippocampal volume almost 10% greater than their peers whose parents showed fewer nurturing behaviors. The hippocampus affects cognitive functioning and emotion regulation.
Unfortunately, parental nurturing did not effect the hippocampal volume of children with early-onset depression.
Dr. Luby and colleagues think their findings could have “profound public health implications and suggest that greater public health emphasis on early parenting could be a very fruitful social investment.”
“The finding that early parenting support, a modifiable psychosocial factor, is directly related to healthy development of a key brain region known to impact cognitive functioning and emotion regulation opens an exciting opportunity to impact the development of children in a powerful and positive fashion”.
Parent-Child Therapy Technique Could Be Useful for Depression in Very Young Children
 There are few treatments approved by the Federal Drug Administration for the treatment of depression in very small children. But a new therapeutic technique parents can use with their children is being studied.
There are few treatments approved by the Federal Drug Administration for the treatment of depression in very small children. But a new therapeutic technique parents can use with their children is being studied.
According to an article published by the Brain and Behavior Research Foundation,
Now, a novel approach called Parent Child Interaction Therapy-Emotion Development (PCIT-ED), being tested by Brain & Behavior Research Foundation Independent Investigator Grantee Joan Luby, M.D., and colleagues at Washington University in St. Louis, has shown promise in an early trial of improving mood and behavior in very young children with depression. The results of the pilot study were reported online on Oct. 31, 2011 in the Journal of Child Psychology and Psychiatry.
PCIT-ED is a dyadic psychosocial intervention with two components. The PCIT part is aimed at strengthening the parent-child relationship by teaching positive play techniques and training parents in ways to handle children’s noncompliant and disruptive behavior. PCIT has previously been shown to be effective for treating disruptive disorders among preschoolers. The new ED component was designed to help parents enhance their children’s ability to recognize their own emotions as well as emotions in others and to more effectively regulate intense emotions.
[Editor’s Note.: our emphasis]
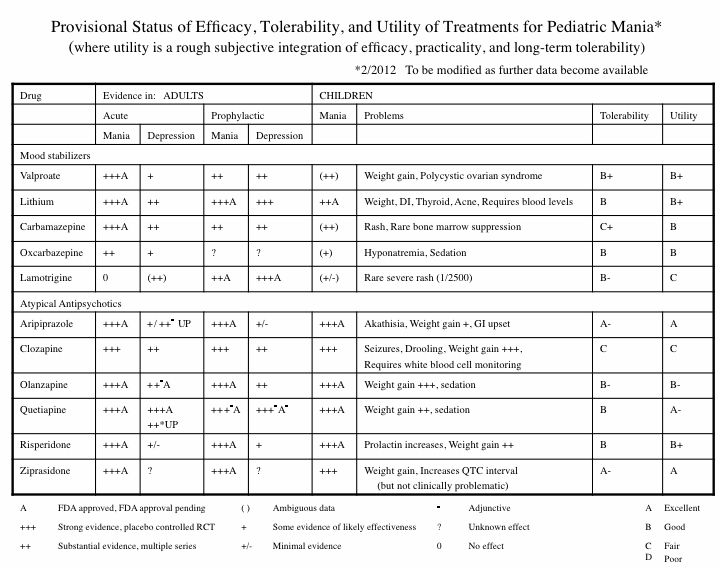
Efficacy, Tolerability, and Utility of Treatments for Pediatric Mania
The following table analyzes the information currently available about various treatment options (including treatments NOT yet FDA approved for the treatment of children):
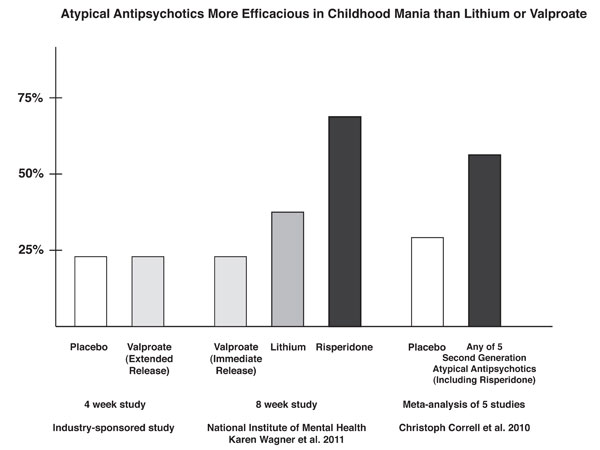
Risperidone Trumps Valproate and Placebo for Treatment of Young Children with Mania
 At another symposium at the annual meeting of the American Academy of Child and Adolescent Psychiatry, Bob Kowatch of Ohio State University discussed a controlled trial of valproate, risperidone, and placebo in children 3 to 7 years of age (average age 5.5) with a diagnosis of bipolar I disorder and a Young Mania Rating Scale score (YMRS) greater than 20 at baseline. All of the children were severely ill with an average Clinical Global Assessment of Severity (CGAS) score of 44. Seventy-six percent had comorbid attention deficit hyperactivity disorder (ADHD) and 15% had an anxiety disorder. Valproate doses started at 10mg/kg and were increased after 4 days to achieve blood levels of 80 to 100µg/ml. The average dose of valproate was 300mg/day and the average blood level was 88 µg/ml. Risperidone was started at 0.25mg and increased as needed. The average dose of risperidone was 0.5mg per day.
At another symposium at the annual meeting of the American Academy of Child and Adolescent Psychiatry, Bob Kowatch of Ohio State University discussed a controlled trial of valproate, risperidone, and placebo in children 3 to 7 years of age (average age 5.5) with a diagnosis of bipolar I disorder and a Young Mania Rating Scale score (YMRS) greater than 20 at baseline. All of the children were severely ill with an average Clinical Global Assessment of Severity (CGAS) score of 44. Seventy-six percent had comorbid attention deficit hyperactivity disorder (ADHD) and 15% had an anxiety disorder. Valproate doses started at 10mg/kg and were increased after 4 days to achieve blood levels of 80 to 100µg/ml. The average dose of valproate was 300mg/day and the average blood level was 88 µg/ml. Risperidone was started at 0.25mg and increased as needed. The average dose of risperidone was 0.5mg per day.
On the main outcome measure of decrease in the YMRS score risperidone was substantially more effective than placebo, while valproate showed only marginal nonsignificant effects. However on the Clinical Global Impressions (CGI) scale for improvement in illness, risperidone showed 87% response, valproate 75% response, and placebo no response. In terms of 50% reduction in the YMRS score, this endpoint was achieved in 88% on risperidone, 50% valproate, and 15% on placebo.
Weight gain was mild on valproate and substantially more on risperidone. Risperidone was also associated with increases in insulin and prolactin.
The effect size (the size of the change the drug brought about in this study, which is calculated by dividing the mean difference between the experimental group and the control group by the standard deviation) for risperidone was extraordinarily large (3.58); very large for valproate (1.66), and moderate for placebo (0.56). The odds of getting well were 5 times greater than placebo for risperidone and 1.9 times greater than placebo for valproate.
Editors note: These data in very young children (aged 3 to 7) resemble other controlled data in the literature about the treatment of older children and adolescents, indicating a superiority of atypical antipsychotics over placebo and a greater magnitude of effect achieved with atypicals than with valproate. Based on these new data and the Federal Drug Administration (FDA) approval of several atypical antipsychotics for children with bipolar illness from ages 10 to 17, Dr. Kowatch recommended a new treatment algorithm for childhood onset bipolar disorder. Read more
Bipolar Disorder and Its Comorbidities in Youth
A symposium on bipolar disorder and its comorbidities in children and adolescents was held at the annual meeting of the American Academy of Child and Adolescent Psychiatry in 2011. The following findings were reported there.
ADHD
Researcher Janet Wozniak discussed the relationship of bipolar illness and attention deficit hyperactivity disorder (ADHD). Based on interviews of family members of children with bipolar illness alone, bipolar illness plus ADHD, ADHD alone, and controls, she concluded that bipolar illness occurred more often in families of children with bipolar illness with or without ADHD. Similarly, she showed that there was more ADHD in relatives of children with either ADHD alone or ADHD comorbid with bipolar illness. She concluded that the comorbidity of bipolar illness and ADHD is a unique subtype of bipolar disorder and requires further study.
Emotional Dysregulation and Substance Abuse
In another presentation, Tim Wilens indicated that those with bipolar disorder and emotional dysregulation had an 8- to 20-fold increased risk of having a substance abuse comorbidity with their bipolar disorder.
Substance Abuse Comorbidity
In a third presentation, Ben Goldstein reported that the onset of bipolar illness predates the onset of substance abuse in 60 to 83% of instances of comorbid illness. He emphasized the dramatic negative impact of comorbid substance use in children with bipolar disorder in terms of increasing legal entanglements, pregnancy, academic failure, suicide, and decreased compliance with medications. He reported that in the multi-site, National Institute of Mental Health (NIMH)-funded Course and Outcome of Bipolar Illness in Youth (COBY) study, the largest longitudinal study to date of youth with bipolar disorder, the risk of new onset substance abuse over the course of 4 years of follow-up was 32%. These data taken with the 15% of children who already had substance abuse at intake indicates that in this study approximately half of the children with bipolar illness had or acquired a substance abuse problem near the beginning of their illness. Two-thirds of the children in the study had abused both alcohol and cannabis. Read more
Obesity and Bipolar Disorder: News from the American Academy of Child and Adolescent Psychiatry
 At the annual meeting of the American Academy of Child and Adolescent Psychiatry in Toronto in October 2011, a symposium on the impact of obesity on the course of childhood onset bipolar illness was held.
At the annual meeting of the American Academy of Child and Adolescent Psychiatry in Toronto in October 2011, a symposium on the impact of obesity on the course of childhood onset bipolar illness was held.
Typical Treatment of Bipolar Disorder in Youth
David Axelson described the typical outcome of bipolar illness and the medications used during naturalistic treatment. The data came from the large collaborative Course and Outcome of Bipolar Illness among Youth (COBY) study, in which he and his colleagues followed 255 patients with bipolar I disorder (BP I), 30 patients with bipolar II (BP II), and 153 patients with bipolar not otherwise specified (BP NOS) for a mean of 5 years. He discussed only BP I children at the symposium.
The study initially followed 270 BP I children for a mean of 582 weeks. They ranged in age from 7 to 17 years (average 14.4 years). Ninety-three percent of the children were treated with one or more antimanic (AM) agents. These included atypical antipsychotics (AA) in 77%, valproate or carbamazepine in 44%, and lithium in 47%. Antidepressants (ADs) were used in 46% of the children, stimulants in 43%, and benzodiazepines in 21%. Sixty percent had been on two classes of antimanic medications concurrently at some point.
A univariate analysis showed that older children received smaller amounts of antipsychotics and more anticonvulsants and lithium. Variables associated with better response, that is, a rating of either much or very much improved on the Clinical Global Impressions scale for bipolar disorder (CGI-BP), included older age and treatment with atypical antipsychotics. Those who had comorbid attention deficit hyperactivity disorder (ADHD) or psychosis at baseline did more poorly. Mean symptom scores were better when the children received any anti-manic treatment including an atypical or lithium, but worse when they received valproate or carbamazepine.
These data are similar to those from other prospective treatment outcome studies in childhood-onset bipolar I illness. Taken together they all suggest that the illness is difficult to treat and stabilize even when multiple medicines are used in combination.
Obesity and Mood Disorders in Youth
Another speaker, Ben Goldstein, indicated that in the scientific literature, obesity has been associated with a higher number of depressive episodes and longer length of depression, more recurrences of depression, more anxiety disorders, increased numbers of hospitalization, more suicide attempts, and worse functional outcomes. In the same group of patients discussed by Axelson above, 42% were overweight or obese, compared to a 34% incidence in the general population of children in this age range.
Factors associated with overweight included substance abuse, a history of physical abuse, prior hospitalization, and being on 2 or more medications. Those who were overweight or obese spent more time ill in a manic or depressive episode. Read more
News From The “TEAM” Study: Risperidone Superior To Valproate And Lithium In Childhood Mania
A symposium at the Annual Meeting of the American Association of Child and Adolescent Psychiatry discussed the Treatment of Early Age Mania (TEAM) study, which comprised 5 different sites in Pittsburgh, Washington DC, Baltimore, St. Louis, and Cleveland. This randomized partially blinded study compared risperidone, valproate, and lithium for the treatment of children with bipolar I mania.
Participants were all severely ill with a Clinical Global Assessment of Severity score (C-GAS) of less than 60 (the mean was 39, indicating that the children were substantially impaired). More than three quarters had psychosis (i.e. hallucinations or delusions) and 99% had dramatic mood shifts within a day (ultradian cycling). All the children had the cardinal symptom of elevated mood.
Among the 290 participants, there was a high incidence of Axis I comorbidities, with 98% of patients having a disruptive behavioral disorder, 77.3% an anxiety disorder, 31% some form of sleep disturbance, and 17% an elimination disorder, of which 15% had enuresis (bedwetting). Nightmares were present in 25.9% of the sample, sleepwalking in 7.2%, and night terrors in 4.8%.
For the purposes of the study, response was considered to have been achieved when a child received a rating of 1 (not ill) or 2 (minimally ill) on the Clinical Global Impressions scale modified for bipolar illness (CGI-BP).
The children (age 6 to 15 with a mean age of 11) were randomized to treatment for 8 weeks with lithium, valproate, or risperidone. Lithium treatment reached blood levels of 1.1 to 1.3mEq/L, valproate reached levels of 111 – 225µg/ML, and risperidone doses were up to 3mg per day. Children who were taking psychomotor stimulants for treatment of ADHD remained on the stimulants after randomization to one of the three drugs. While the treating physicians and clinicians were not blind, blind ratings were performed at week 8.
With a response rate of 68.5%, risperidone was superior to lithium (35.6%) and valproate (24%) based on CGI-BP scores. The mean dose of risperidone was 2.6mg +/- 1.2 per day. The mean blood level at week 8 for lithium was 1.1mEq/L and for valproate was 114µg/ML.
The number of children who improved sufficiently for their C-GAS scores to rise above 60 was also greater for risperidone at 48.3% compared to lithium at 26.7% and valproate at 17.0%. Read more