Study Finds No Substantial Risk of Infant Cardiac Problems from Antidepressant Use During Pregnancy
In the past there has been some concern that selective serotonin reuptake inhibitor (SSRI) antidepressants taken during pregnancy could increase an infant’s risk of cardiac problems. There was particular concern that the SSRI paroxetine could lead to right ventricular outflow tract obstruction, and sertraline could lead to ventricular septal defects. A 2014 study by KF Huybrechts et al. in the New England Journal of Medicine analyzed data from 949,504 women in a Medicaid system from three months before pregnancy until one month after delivery during the years 2000-2007.
Infants born to mothers who had taken antidepressants during their first trimester were compared to infants whose mothers had not taken antidepressants. In total, 6.8% or 64,389 women had used antidepressants in their first trimester.
While the rate of cardiac defects in newborns was greater among those mothers who had taken antidepressants (90.1 infants per 10,000 infants who had been exposed to antidepressants versus 72.3 infants per 10,000 infants who had not been exposed to antidepressants), this relationship diminished as confounding variables were removed. The relative risk of any cardiac defect after taking SSRIs was 1.25, but this decreased to 1.12 when restricted to only those mothers who were diagnosed with depression, and to 1.06 when the researchers controlled for things like depression severity. (All relative risk numbers were calculated with a 95% confidence interval.)
The researchers concluded that there is no substantial risk of increased cardiac defects in children born to mothers who took antidepressants during their first trimester.
Antidepressant Use Dropping in Bipolar Disorder in Spain
In the clinic of researcher Eduard Vieta in Barcelona, a study was recently completed showing that antidepressant use in patients with bipolar disorder (where antidepressants are not effective) had dropped from around 50-60% in 2007 (in Baldessarini’s study) to about 30% in 2013 and 2014, and conversely lithium, anticonvulsants, and atypical antipsychotics, which have much more evidence of efficacy, were all used much more often, or about 60% of the time.
Editor’s Note: Hopefully these data from Spain will soon be matched by similar data in the US showing that evidenced-based treatments for bipolar depression are in fact being used instead of antidepressants, which can have adverse effects, such as switching into mania or cycle acceleration.
FDA Warning About Antidepressants Followed by Drop in Use, and Increase in Suicide Attempts
A decade ago the Federal Drug Administration (FDA) released several warnings that children, adolescents (ages 10–17), and young adults (ages 18-29) taking antidepressants were at increased risk for suicidal ideation and behavior. A recent study found that following these warnings, antidepressant use among adolescents, young adults, and adults dropped, and psychotropic drug poisonings (a validated measure of suicide attempts) increased among adolescents and young adults. Numbers of completed suicides did not change for any age group.
The decision to place the warnings on antidepressant packaging was somewhat controversial because it was based on studies that were not necessarily designed to measure suicide risk. The relationship between depression, medication, and suicide is complicated. Medication can improve mood, but patients may seek out medication because of pre-existing suicidal thoughts, and the medication may not reduce these in young people.
The reduction in antidepressant use that occurred after the warnings was accompanied by a drop in depression diagnoses in children and adults. Studies have suggested that the decreases in antidepressant were not accompanied by increases in other treatments, such as psychotherapy or atypical antipsychotics, among young people. Increased monitoring of patients was called for in the FDA’s box warning, but did not take place.
The study of the aftermath of the FDA warnings, published by Christine Y. Lu et al. in a 2014 article in the journal BMJ, used data from 11 insurance networks throughout the US. The researchers used an interrupted time series study design, which is used to show whether a policy causes an abrupt change in the expected slope of study outcomes. Data covered the pre-warning period (first quarter of 2000 to third quarter of 2003), the warning “phase-in” period (last quarter of 2003 to last quarter of 2004) and the post-warning period (first quarter of 2005 to last quarter of 2010). The study cohorts included around 1.1 million adolescents, 1.4 million young adults, and 5 millions adults per quarter.
Among adolescents, the previously upward trend in antidepressant use declined by 31.0% in the second year after the warnings, and psychotropic drug poisonings increased by 21.7% (a figure that was statistically significant for males). Poisonings by any drug increased by 13.9% in the second year after the warnings. After 2008, the downward trend in antidepressant use reversed, indicating that either the initial effects of the warning had worn off or that modifications to the warnings in May 2007, which encouraged patients and doctors to consider the risk of antidepressants alongside the risk of leaving mood disorders untreated, led to increased use.
Among young adults, the upward trend in antidepressant use declined by 24.3% in the second year after the warnings, and psychotropic drug poisonings increased by 33.7%, a statistically significant change for both male and female patients.
Among adults, to whom the warnings were not directed, antidepressant use decreased by 14.5% in the second year after the warnings.
The study by Yu et al. is the first to show that suicide attempts actually increased after the FDA warnings. The authors suggest that the increase in suicide attempts might be a consequence of undertreating mood disorders, since antidepressant use dropped simultaneously. The warnings and related media attention may have led to these unintended consequences, since media reports can sometimes be oversimplified.
CRP, A Readily Available Marker of Inflammation, Predicts Response To Two Antidepressants
C-reactive protein, or CRP, is a protein found in blood plasma, the levels of which rise in response to inflammation. In a recent study, levels of CRP were able to predict which of two antidepressants a patient was more likely to respond to.
The 2014 article by Rudolph Uher et al. in the American Journal of Psychiatry reported that low levels of CRP (<1 mg/L) predicted a good response to the selective serotonin reuptake inhibitor (SSRI) escitalopram (Lexapro) while higher levels of CRP predicted a good response to the tricyclic antidepressant nortriptyline, a blocker of norepinephrine reuptake.
The research was part of the Genome-Based Therapeutic Drugs for Depression (GENDEP) study, a multicenter open-label randomized clinical trial. CRP was measured in the blood of 241 adult men and women with major depressive disorder. In the article the researchers say that CRP and its interaction with medication explained more than 10% of the individual variance in response to the two antidepressants.
If these findings can be replicated with these and similarly acting drugs, it would be a very large step in the direction of personalized medicine and the ability to predict individual response to medications.
Traditional Antidepressants Are Not Effective in Bipolar Depression
 Bipolar illness affects 4.5% of the US population. According to researcher Kathleen Merikangas, 1.0% have bipolar I disorder, 1.1% have bipolar II disorder, and the remainder have subthreshold symptoms. Mark Frye, Chairman of the Department of Psychiatry at the Mayo Clinic, gave a lecture on antidepressants in bipolar illness at the 2014 meeting of the American Psychiatric Association.
Bipolar illness affects 4.5% of the US population. According to researcher Kathleen Merikangas, 1.0% have bipolar I disorder, 1.1% have bipolar II disorder, and the remainder have subthreshold symptoms. Mark Frye, Chairman of the Department of Psychiatry at the Mayo Clinic, gave a lecture on antidepressants in bipolar illness at the 2014 meeting of the American Psychiatric Association.
The newest data from meta-analyses indicate that traditional antidepressants that are effective in unipolar depression are not effective in bipolar depression. Some patient groups, especially those with very early onset depression and mixed depression, are at increased risk of switching into mania and making a suicide attempt while taking antidepressants.
Unipolar depressed patients with a genetic variation that produces a short form of the serotonin transporter (5HT-LPRs/s) are at increased risk for depression in adulthood following a history of childhood adversity, and tend to respond less well to antidepressants. Frye found that 5HT-LPRs/s is weakly associated with switching into mania when antidepressants are given to patients with bipolar depression.
At the same symposium, researcher Mike Gitlin reviewed data on combination therapy, which is rapidly becoming the norm, indicating that in most circumstances, it is superior to monotherapy.
Researcher David Miklowitz reviewed the impressive data on the superiority of most forms of targeted psychotherapy or psychoeducation compared to treatment as usual for bipolar depression. He noted his own finding that Family Focused Therapy (FFT) not only is effective in adolescents and adults with bipolar disorder, but also in reducing illness and dysfunction in those with prodromal disorders (such as depression, cyclothymia, and bipolar not otherwise specified) in situations where there is a family history of bipolar disorder.
Eight components of FFT are:
- Recognition of prodromal symptoms and development of treatment strategies for them.
- Recognition and management of stress and triggers using cognitive restructuring.
- Development of a relapse prevention plan and rehearsal of what to do.
- Regularization of sleep.
- Encouragement of treatment adherence with an eye to a good future.
- Enhancement of emotional self-regulation skills, including cognitive restructuring.
- Improvement of family relationships and communication.
- Education about substance abuse avoidance and treatment for that and other comorbidities.
Many of these are also key components of group psychoeducation, cognitive-behavioral therapy, and interpersonal and social rhythms therapy, and all of these are effective in treating and preventing bipolar depression compared to treatment as usual. It is noteworthy that in the research of Francesc Colom, 90% of patients randomized to treatment as usual relapsed within 24 months, while psychoeducation was highly effective in preventing relapses over the next five years.
This editor (Robert M. Post), the discussant for the symposium, emphasized that the main take-away messages of the speakers were: use more lithium, use more caution and fewer antidepressants in treating bipolar depression, use more combination therapy for acute illness and for maintenance, and definitely use more psychotherapy. Read more
Acetly-l-carnitine May Be Effective in Treatment-Resistant Depression
 Not all patients with unipolar depression respond to the currently available antidepressants. Acetyl-l-carnitine is a compound that enhances mitochondrial function and neuroplasticity and is effective in the treatment of peripheral neuropathy (damage to the peripheral nerves, which sometimes occurs in chemotherapy or diabetes). It is now being investigated as an antidepressant for patients who have not responded to typical antidepressants.
Not all patients with unipolar depression respond to the currently available antidepressants. Acetyl-l-carnitine is a compound that enhances mitochondrial function and neuroplasticity and is effective in the treatment of peripheral neuropathy (damage to the peripheral nerves, which sometimes occurs in chemotherapy or diabetes). It is now being investigated as an antidepressant for patients who have not responded to typical antidepressants.
According to a review of the treatment by S.M. Wang et al. published in the Journal of Psychiatric Research in 2014, acetyl-l-carnitine treated depression better than placebo did in four randomized clinical studies. It was better than placebo and equally as effective as the antidepressant fluoxetine and the atypical antipsychotic amisulpride in various studies of dysthymic disorder. It also improved depressive symptoms in people with fibromyalgia and minimal hepatic encephalopathy (liver damage). The usual dose of acetyl-l-carnitine is 1 to 2 grams/day.
Editor’s Note: The role acetyl-l-carnitine will play in treating people with treatment-resistant unipolar or bipolar depression remains to be better clarified.
A Possible Explanation for Vitamin D’s Antidepressant Effects
 Vitamin D plays an important role in the nervous system, regulating the production of neurotrophins, calcium channels, and calcium binding proteins, and it may have antidepressant effects. Researchers are learning more about how the vitamin’s effects take place.
Vitamin D plays an important role in the nervous system, regulating the production of neurotrophins, calcium channels, and calcium binding proteins, and it may have antidepressant effects. Researchers are learning more about how the vitamin’s effects take place.
At the 2014 meeting of the International Society for Bipolar Disorders, Yilmazer et al. reported that vitamin D treatment increased the production of glia-derived neurotrophic factor (GDNF). Neurotrophins like GDNF enhance the survival and growth of neurons. Since other neurotrophins (i.e. brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEG-F)) are low in depression, vitamin D’s effect on GDNF could be important to its antidepressant effects.
Another Blocker of Glutamate Receptor Function with Rapid Antidepressant Effects
Certain drugs such as ketamine and memantine that work by blocking activity at the NMDA receptor for the excitatory neurotransmitter glutamate have antidepressant effects. D-cycloserine is a drug that has a related mechanism and is being studied as an antidepressant. At high doses the drug acts as an antagonist at the glycine site of the NMDA receptor, blocking glycine’s ability to facilitate glutamate transmission through the receptor.
Joshua Kantrowitz, a researcher at Columbia University, reported at a recent scientific meeting that the rapid-onset antidepressant effects of D-cycloserine could be maintained for eight weeks. Similar findings were published in the Archives of General Psychiatry in 2010 and were reported in another study by Uriel Heresco-Levy in a 2013 article in the Journal of Neuropsychopharmacology.
Glutamate is the major excitatory neurotransmitter in the brain and is important for the development of long-term memory. However, glutamate overactivity may contribute to depression. Decreasing this overactivity (with ketamine, memantine, or D-cycloserine) may produce antidepressant effects.
More Evidence That Regular Antidepressants Do Not Work in Bipolar Depression
Psychiatrists most commonly prescribe antidepressants for bipolar depression, but mounting evidence shows that the traditional antidepressants that are effective in unipolar depression are not effective in bipolar disorder. At the 2013 meeting of the American Psychiatric Association, researcher Jessica Lynn Warner reported that among 377 patients with Bipolar I Disorder who were discharged from a hospital, those who were prescribed an antidepressant at discharge were just as likely to be remitted for a new depression than those not given an antidepressant.
The average time to readmission also did not differ across the two groups and was 205 +/- 152 days. Those patients prescribed the serotonin and norepinephrine reuptake inhibitor (SNRI) drug venlafaxine (Effexor) were three times more likely to be readmitted than those not prescribed antidepressants.
These naturalistic data (generated from observations of what doctors normally do and information in the hospital’s clinical notes) resemble those from controlled studies. In the most recent meta-analysis of antidepressants in the treatment of bipolar depression (by researchers Sidor and MacQueen), there appeared to be no benefit to adding antidepressants to ongoing treatment with a mood stabilizer over adding placebo. Randomized studies by this editor Post et al. and Vieta et al. have shown that venlafaxine is more likely to bring about switches into mania than other types of antidepressants such as bupropion or selective serotonin reuptake inhibitors (SSRIs).
In addition, a naturalistic study published by this editor Post et al. in the Journal of Clinical Psychiatry in 2012 showed that the number of times antidepressants were prescribed prior to a patient’s entrance into a treatment network (the Bipolar Collaborative Network) at an average age of 40 was related to their failure to achieve a good response or a remission for a duration of at least six months during prospective treatment.
Editor’s Note: Antidepressants are still the most widely used treatments for bipolar depression, and their popularity over more effective treatments (mood stabilizers and some atypical antipsychotics) probably contributes to the fact that patients with bipolar disorder receiving typical treatment in their communities spend three times as much time in depressions than in manic episodes. Using other treatments first before an antidepressant would appear to do more to prevent bipolar depression. These treatments include mood stabilizers (lithium, lamotrigine, carbamazepine, and valproate); the atypical antipsychotics that are FDA-approved for monotherapy in bipolar depression, lurasidone (Latuda) and quetiapine (Seroquel); and the combination of olanzapine and fluoxetine that goes by the trade name Symbiax.
Evidence from several sources suggests that the SNRI venlafaxine may be a risk factor for switches into mania and lead to re-hospitalizations. Other data suggest that in general, in bipolar depression, augmentation treated with antidepressants should be avoided in several cases: in childhood-onset bipolar depression, in mixed states, and in those with a history of rapid cycling (4 or more episodes per year).
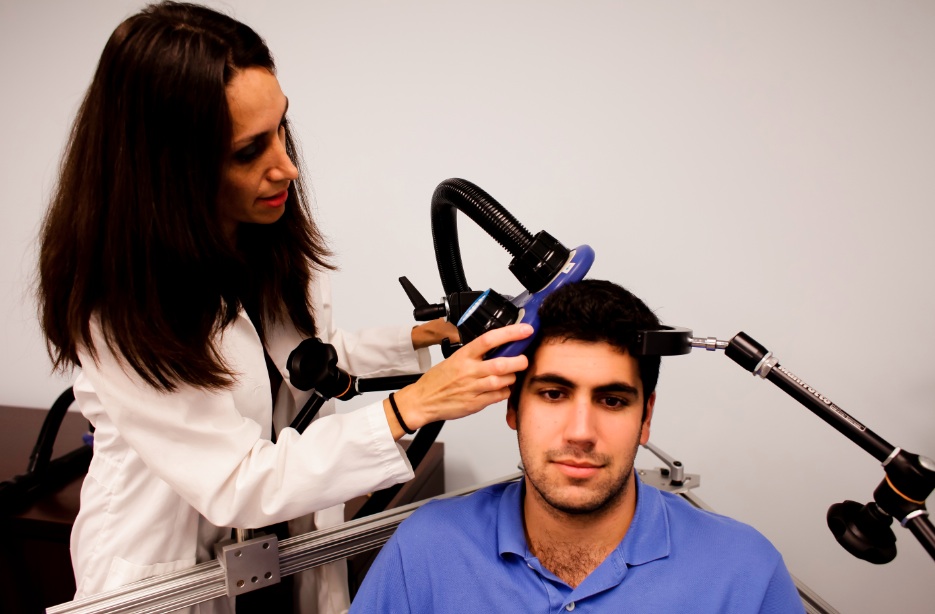
RTMS in Depression: Positive Effects in Long-Term Follow-Up
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive treatment that uses a rapidly changing magnetic field to target neurons, creating a weak electric current. It is used to treat depression, strokes, and other neurological and psychiatric conditions. At the 2013 meeting of the American Psychiatic Association (APA), researcher Linda L. Carpenter reported on new findings about the long-term effects of rTMS.
In the new research, which was led by Mark Andrew Demitrack, 307 patients with unipolar depression who had not responded well to previous antidepressant treatment were given rTMS. They were treated in 43 different clinical practices and administered rTMS according to their evaluating physician, following FDA guidelines. Of the 307 patients who began the study, 264 benefited from initial treatments with rTMS, were tapered off rTMS, and agreed to participate in a one-year follow-up period, by the end of which 68% had improved and 40.4% had achieved complete remission as measured by the Clinical Global Impressions scale for severity of illness.
However, some study participants (30.2%) had worsened by the first follow-up assessment at the 3-month mark, and rTMS had to be re-introduced.