Inflammation and Depression: Treatment Implications
 Vladimir Maletic of the University of South Carolina School of Medicine Greenville gave a plenary talk at the 2018 meeting of the North Carolina Psychiatric Association that described a variety of ways that inflammation can drive depression.
Vladimir Maletic of the University of South Carolina School of Medicine Greenville gave a plenary talk at the 2018 meeting of the North Carolina Psychiatric Association that described a variety of ways that inflammation can drive depression.
Maletic explained that stress can increase neurotransmitters that activate brain macrophages, increase NFkB (a protein that controls DNA transcription and cell survival), and increase brain inflammation, evidenced by elevated levels of the inflammatory markers IL-1b, IL-6, TNF-alpha, and C-reactive protein (CRP). These signs of inflammation are associated with changes in brain function and connectivity that are consistent with depression, fatigue, and cognitive slowing.
Inflammation measured outside of the brain and spinal cord is associated with increased activity of the insula (a key brain sensor and modulator of emotions), disconnection between the prefrontal cortex and the reward circuits in the nucleus accumbens, and decreased function and structural changes to the hippocampus (critical for memory).
Maletic also explained that inflammation changes the way the amino acid tryptophan is metabolized. Normally tryptophan is converted into kyneurenic acid, which prevents excitotoxicity and has anticonvulsant effects. Stress can lead to tryptophan being metabolized instead into quinolinic acid, which is neurotoxic and has been linked to certain psychiatric disorders and neurodegenerative processes. This in turn impairs synaptic functioning, including increasing glutamate and decreasing brain-derived neurotrophic factor (BDNF), impairing a type of glia called oligodendroglia (which produce myelin), and the formation of new neural connections.
These findings have several important implications for treatment. Increases in inflammation have been linked to the atypical type of depression characterized by increased appetite, weight gain, and increased sleep rather than the more classic presentation of depression that includes loss of appetite, weight loss and insomnia. Thus, weight gain, waist circumference, and body mass index (BMI) are correlated with inflammation and can signal a poor response to medications (including the rapid-acting antidepressant ketamine and some other antidepressants). If someone with unipolar depression has high levels of CRP, they tend to have a poorer response to selective serotonin reuptake inhibitor (SSRI) antidepressants, and may respond better to the noradrenergic tricyclic antidepressant nortryptyline, the serotonin and norepinephrine reuptake inhibitors (SNRIs), and the dopamine active antidepressant bupropion.
There is some good news. Read more
Supplements for the Treatment of Schizophrenia
At the 2018 meeting of the North Carolina Psychiatric Association, researcher Karen Graham reviewed evidence for adjunctive treatments that may help treat schizophrenia when added to antipsychotic medications.
Graham endorsed omega-3-fatty acids, saying that they may delay the conversion to schizophrenia in young people at high risk for the illness. Data in chronic schizophrenia are more equivocal.
Data on the effects of vitamin D3 in schizophrenia are mixed, but D3 is often low in patients with psychotic disorders, and supplementation with vitamin D3 in the general population has been associated with decreases in cancer and all-cause mortality.
Graham indicated that in three studies vitamin B6 (pyridoxine) decreased tardive dyskinesia, a side effect of antipsychotic medication that is characterized by repetitive or jerky involuntary movements of the face and body. B6 also reduced the severity of akathisia or restless legs, which is comparable to the effects of 40mg/day of the beta blocker drug propranolol. Graham recommended a dose of 300mg/day of B6 that could be increased up to 600mg twice per day. The onset of effects usually begins by week three, and the cost ranges from 25 to 80 cents per day.
The antioxidant supplement N-acetylcysteine (NAC) may also help. Graham described six studies that found NAC had positive effects on negative symptoms (apathy, blunted emotions, etc.) and/or cognition in patients with schizophrenia. The dosage in these studies was usually 2 grams/day for 24 weeks. The cost was 50 cents per day.
Two 8-week trials of L-theanine (an amino acid found in green and black tea) at doses of 400mg/day improved negative symptoms and anxiety in 40 patients with schizophrenia. The rationale for the study was that L-theanine increases inhibitory neurotransmitters, modulates the amino acid 5-HTP and the neurotransmitter dopamine, increases brain-derived neurotrophic factor (BDNF), and may be neuroprotective after a heart attack or a traumatic brain injury. The cost is 40 cents per day.
Graham reported that the supplement ginkgo biloba produced significant improvement in negative symptoms and total symptoms in eight clinical trials that included a total of 1,033 patients with schizophrenia. Doses ranged from 240 to 360 mg/day. These supplements (usually extracted from leaves of the ginkgo tree) have not been found to have many side effects, but they can reportedly increase post-operative bleeding. Gingko biloba supplements cost 20 to 80 cents per day. There is also at least one positive study of ginkgo biloba in tardive dyskinesia.
Three of four studies of cannabidiol in schizophrenia have been positive (at doses of 600, 800, and 1,000 mg/day in studies that lasted four to six weeks). There are now six additional ongoing studies listed on the website clinicaltrials.gov. There is little of this diol component in regular marijuana, and the cost of pure cannabidiol is unfortunately an exorbitant $60 to $100/day.
There is a positive controlled study of the herb ashwagandha in 66 patients with schizophrenia.
Not included in Dr. Graham’s review was the prenatal treatment of women with phosphatidylcholine (900mg/day) followed by supplements in the newborn, which normalized an aspect of sensory gating known as P50 in patients with schizophrenia. Healthy individuals show a reduced response to an auditory cue when it is repeated 50 milliseconds after the initial cue. In people with schizophrenia, response to the repeated cue is not suppressed. This has been suggested by researchers Robert Freedman and Randal G. Ross in a 2015 article in the Shanghai Archives of Psychiatry as a possible primary preventive approach to schizophrenia.
Pregnant women in their second and third trimesters should at least consume foods high in choline, especially if the fetus is at high risk for schizophrenia because of a family history of schizophrenia.
Beef liver is very high in choline, providing 420mg per slice. Other animal products provide significant choline, such as eggs (120 mg/egg), beef (90mg/100g), chicken liver (85mg/liver), fish (85mg/100g), bacon (35mg/strip) or other pork, chicken (67mg/100g). Tofu (36mg/half cup) and cereal (22mg/half cup) are also sources of choline.
Foods High in Choline
| Beef liver | 1 slice | 420mg choline; |
| Egg | 1 egg | 120; |
| Beef | 100 gm | 90; |
| Chicken liver | 1 liver | 85; |
| Fish | 100 gm | 85; |
| Bacon or pork | 2 strips bacon | 70; |
| Chicken | 100 gm | 67; |
| Tofu | 120 ml (0.5 cup) | 36; |
| Cereal | 120 ml (0.5 cup) | 22 |
Using Light to Improve Sleep and Depression
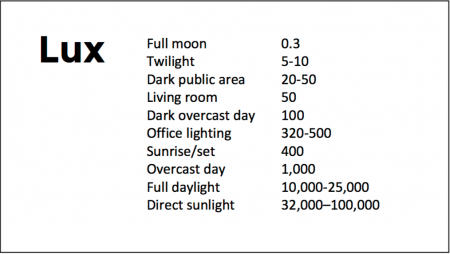
At the 2018 meeting of the North Carolina Psychiatric Association, researcher Chris Aiken described the phenomenon of sleep inertia, when people are awakened from deep sleep by an alarm, rather than waking at the end of a sleep cycle, and are groggy for 15 minutes. Depressed people may stay groggy for 4 hours. A dawn simulator may help. These lights turn on gradually over the course of 30 to 60 minutes, reaching 250 lux while the patient is still asleep. Dawn simulators have worked in eight out of ten controlled clinical trials to help people with seasonal affective disorder, adolescents, and normal adults wake up more easily. They range in cost from $25 to $90 and some brands include PER2LED or LightenUp. Aiken says dawn simulators can improve depression, sleep quality, and cognition.
Evening and nighttime light: Bright lights and blue light, like the light that comes from electronic screens, can shut down the body’s secretion of melatonin, making people awake and alert in the evening when they should be getting sleepy. Dim light or glasses that filter out blue light allow increases in melatonin secretion in the evening, while bright light suppresses it. Missing this early melatonin pulse creates “night owls” who have delayed sleep onset.
Because light still reaches our eyes through our eyelids as we sleep, even low-level light during the night impairs sleep, cognition, and learning, and increases the risk of depression by a hazard ratio of 1.8 (about double the risk). A 2017 study by Kenji Obayashi in the American Journal of Epidemiology found that bedroom light above 5 lux elevated rates of depression in older adults after two years of followup. Living room light averaged around 50 lux and increased depression further.
The treatment is turning off TVs, electronic screens, and cellphones in the evening or wearing blue-blocking glasses, which can be found for less than $10. Blue-blocking glasses can increase calmness and reduce anxiety, and even are effective in treating mania. Then, during sleep, wear an eye mask or get light-blocking blinds or curtains for windows. For a complete blackout, use blackout curtains, aluminum foil over windows, electric tape over LED lights, or try sleeping in the basement.
Aiken suggests that to re-instate healthy sleep patterns, people should institute virtual darkness from 6pm to 8am, including wearing blue-blocking glasses when out of bed. Then they should institute total darkness or wear an eye mask when in bed. When symptoms improve, this routine can gradually be shifted to begin later in the evening, such as two hours before bedtime.
Blue light filters are also available for smartphones and tablets including Apple Nightshift mode, Kindle BlueShade, and Android Twilight and Blue Light Filter.
Glasses that filter out blue light include Uvex Ultraspec 2000, 50360X ($7 on Amazon) and Uvex Skyper 351933X ($7-10 on Amazon). The website lowbluelights.com sells blue-blocking glasses from $45 and a variety of other blue-free lighting products such as lightbulbs and flashlights.
Bright light therapy for unipolar and bipolar depression: 30 minutes of bright light (7,500 to 10,000 lux) in the morning can help treat depression in unipolar and bipolar disorder and seasonal affective disorder. The effects usually take 3 to 7 days to set in, but they only last while a patient continues using the bright light in the morning. Researcher Dorothy K. Sit and colleagues found that bright light therapy in the morning sometimes caused hypomanic reactions in people with bipolar disorder, and reported in a 2018 article in the American Journal of Psychiatry that midday light therapy (from noon to 2:30pm) was also effective without this unwanted effect. However, a 2018 article by Ne?e Yorguner Küpeli and colleagues in the journal Psychiatry Research suggested that a half hour of morning light for two weeks was sufficient to bring about improvement in 81% of patients with bipolar disorder and did not cause serious side effects.
Melatonin regimen for sleep onset delay: Melatonin can be used to treat severe night-owls with a very late onset of sleep (for example, going to bed at 2 or 3am and sleeping late into the morning). Melatonin can help with sleep onset to some extent when used at bedtime, but in those with an extreme phase shift, researcher Alfred J. Lewy recommends a regimen of low dose priming with 400–500 micrograms of melatonin at 4pm and then a full dose of 3 milligrams of melatonin at midnight. The 4pm priming dose helps pull back the delayed onset of the body’s secretion of melatonin toward a more normal schedule.
Recent Cannabis Use Linked to Greater Symptoms of Anxiety and Mood Disorders and Less Response to Treatment
 In a 2018 systematic literature review published in the Journal of Clinical Psychiatry, researcher George Mamman and colleagues reported that across 12 studies of people with anxiety and mood disorders, participants who had used cannabis in the previous six months had more symptoms than those who had used less cannabis or no cannabis during that period.
In a 2018 systematic literature review published in the Journal of Clinical Psychiatry, researcher George Mamman and colleagues reported that across 12 studies of people with anxiety and mood disorders, participants who had used cannabis in the previous six months had more symptoms than those who had used less cannabis or no cannabis during that period.
The 12 studies reviewed included a total of 11,959 participants. Four studies looked at post-traumatic stress disorder (PTSD), one at panic disorder, five at bipolar disorder, and 2 at depressive disorder. In addition to finding that recent cannabis use was associated with greater symptoms, the authors of the review also found that in 10 of the 12 studies, recent cannabis use was associated with less symptom improvement in response to treatment for bipolar disorder, depression, and PTSD; including both medication and psychotherapy.
In bipolar disorder, cannabis use was associated with greater symptom severity. Cannabis use for more than one year was linked to more recurrences of mania and shortened time to a recurrence. Compared to participants with no prior use of cannabis, those with a cannabis use disorder had more depressive symptoms, including sleep troubles and loss of interest in activities one had previously enjoyed.
In PTSD, any cannabis use at the beginning of the analysis period and sustained use of cannabis over time were both linked to greater symptom severity in the four months following the beginning of the analysis.
Mammen and colleagues cautioned that these results are limited based on the differences in measurements across the 12 studies, the inpatient populations under study, and the uncontrolled nature of the cannabis the participants accessed on their own time. However, the authors suggest that the findings may inform patients’ and doctors’ conversations about whether or not to use cannabis.
Marijuana Use in Early Adolescence Triples Risk of Psychosis At Age 18
 Hannah J. Jones and colleagues reported in the journal JAMA Psychiatry in 2018 that early- and late-onset marijuana use increased the risk of psychosis at age 18 (odds ratio 3.7 to 2.97). Interestingly, early-onset cigarette use also increased risk of psychosis, but much of the link between cigarette use and psychosis disappeared after correcting for confounding variables.
Hannah J. Jones and colleagues reported in the journal JAMA Psychiatry in 2018 that early- and late-onset marijuana use increased the risk of psychosis at age 18 (odds ratio 3.7 to 2.97). Interestingly, early-onset cigarette use also increased risk of psychosis, but much of the link between cigarette use and psychosis disappeared after correcting for confounding variables.
The data on 5,300 participants born from 1991 to 1992 came from the Avon Longitudinal Study of Parents and Children. Researchers followed up with the participants about their use of marijuana and cigarettes at least three times between the ages of 14 and 19.
Editor’s Note: These data add to a host of epidemiological data that smoking marijuana doubles the risk of psychosis. Risk is further increased among people with a common genetic variant (val/val) of the gene for COMT (catechol-O-methyltransferase), which metabolizes prefrontal dopamine. The variant, which includes two valine amino acids, functions better than other variants that include methionine amino acids. People with val/met or met/met COMT genes metabolize dopamine more slowly, making them relatively protected.
The data are also pretty strong that early heavy use of marijuana is a risk factor for new onset of both bipolar disorder and schizophrenia (and not just an earlier onset in those who might have been vulnerable otherwise).
While marijuana use has become more mainstream with its legalization in many states, its recreational use still carries risks of mental illness. In addition to increasing psychosis risk, marijuana use can also make bipolar disorder more difficult to treat.
A minor component of marijuana, cannabidiol, can have some positive effects, but what you get most of when consuming marijuana is tetrahydrocannabinol (THC), which produces symptoms that resemble psychosis.
Data in rats indicate that a father rat’s use of THC as an adult increases the risk that his offspring (with which he has no contact) will be prone to opiate addiction. The effect is an epigenetic one, conveyed by chemical changes in the father’s DNA that get passed on to the next generation via changes that persist in his sperm. We don’t know if this also happens with humans. So even if you are not worried about your own health, avoiding marijuana use might be good for your children.
Pilot Study Finds Intravenous Ketamine Improves Tough-to-Treat Adolescent Depression
A 2018 open study by Kathryn R. Cullen and colleagues in the Journal of Child and Adolescent Psychopharmacology suggests that intravenous ketamine may improve depression in adolescents who have not responded to at least two antidepressants.
Thirteen patients ranging in age from 12 to 18 with treatment-resistant depression were given six ketamine infusions over a period of two weeks, at doses of 0.5 mg/kg of body weight. A 50% drop in scores on the Children’s Depression Rating Scale-Revised (CDRS-R) was considered a good response, and the average drop in participants’ scores was 42.5%. Five of the thirteen participants (38%) met the criteria for a good response. Three of these participants were still in remission at six weeks, while the other two relapsed within two weeks.
Ketamine was fairly well-tolerated by the young participants. Some had temporary dissociative symptoms or blood pressure changes. Higher absolute doses of ketamine were linked to better response.
The response rates in this group were not as good as in some studies of adults. More research using larger sample sizes and placebo controls is needed to optimize dosing and clarify the safety and efficacy of intravenous ketamine in adolescents with tough-to-treat depression, but this is a promising finding in a small number of adolescents.
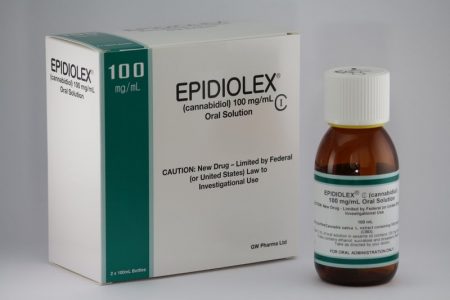
Cannabidiol Drug Approved For Rare and Severe Types of Epilepsy
In June, the US Food and Drug Administration (FDA) for the first time approved a drug derived completely from the cannabis plant. The drug, Epidiolex, a syrup, contains cannabidiol, the cannabis component that has been found to treat certain ailments. In a news release, the FDA stated that cannabidiol does not cause intoxication or a ‘high’. Tetrahydrocannabinol, or THC, is the cannabis component that makes people high, impairs cognition, and can induce paranoia.
The approval led, in September, to the Drug Enforcement Agency re-classifying FDA-approved drugs containing cannabidiol derived from cannabis and less than 0.1% THC as schedule V controlled substances. So far only Epidiolex meets these criteria. Cannabis had previously been classified as a schedule I controlled substance, in the same legal category as heroin, LSD, or ecstasy.
Epidiolex is now approved to treat two rare, severe types of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, in patients aged two years and older. It is the first FDA-approved treatment for Dravet syndrome, a genetic condition that appears in the first year of life when babies develop fever-related seizures. Other types of seizures, even including a continuous seizure state, can occur later.
Lennox-Gastaut syndrome also develops in young children, usually between the ages of three and five. They have multiple types of seizures with debilitating consequences.
In three randomized, double-blind, placebo-controlled clinical trials that included a total of 516 patients with either Lennox-Gastaut syndrome or Dravet syndrome, the drug reduced the frequency of patients’ seizures compared to placebo.
Side effects of Epidiolex include sleepiness, sedation and lethargy; elevated liver enzymes; decreased appetite; diarrhea; rash; fatigue, malaise and weakness; insomnia, sleep disorder and poor quality sleep; and infections. The FDA also warned in its approval that “[a]s is true for all drugs that treat epilepsy, the most serious risks include thoughts about suicide, attempts to commit suicide, feelings of agitation, new or worsening depression, aggression and panic attacks.” The drug is produced by GW Pharmaceuticals, which has already gained approval outside the US for a cannabis-based drug to treat multiple sclerosis.
Editor’s Note: It is important for readers to know that most marijuana available in the US contains mostly THC with minimal cannabidiol.
Early Intervention Works in Schizophrenia: Also Needed in Bipolar Disorder
For twenty years, evidence has shown that early intervention can ameliorate many of the adverse consequences of schizophrenia. In a 2018 article in the journal Annual Review of Clinical Psychiatry titled “Transforming the treatment of schizophrenia in the United States: The RAISE Initiative,” Lisa B. Dixon and colleagues described the importance of early intervention in schizophrenia. RAISE stands for Recovery After an Initial Schizophrenia Episode. Dixon and colleagues emphasize that shortening the time that a patient’s psychosis goes untreated, which averages 74 months, is critical to achieving good outcomes. In parallel to these consistent findings, researchers of bipolar disorder (including this editor Robert M. Post and colleagues) have found that an increased length of the interval before treatment is initiated in childhood-onset bipolar disorder is associated with a poor outcome in adulthood.
The RAISE program consists of four interventions: personalized psychopharmacology using a computerized decision support system, individual resilience therapy, family psychoeducation and therapy, and supportive employment and education. Compared with patients receiving standard treatments, patients who participated in the RAISE program showed greater improvements on almost all measures, including the Heinrichs-Carpenter Quality of Life Scale (main outcome), the Calgary Depression Scale for Schizophrenia, the Positive and Negative Syndrome Scale, treatment duration, and engagement in work and school. Moreover, the improvements were more substantial among patients with a shorter duration of untreated psychosis.
Editor’s Note: These findings are of great importance in their own right, but they also have great implications for treatment and research efforts in bipolar disorder. A 2013 randomized study by Lars Kessing and colleagues published in the British Journal of Psychiatry found that in bipolar patients hospitalized for a first or second episode of mania, two years of comprehensive treatment with psychotherapy, pharmacotherapy, and illness education that included mood monitoring and early symptom recognition was vastly superior to typical treatment, and this held true even six years later. In a 2014 article in the Journal of Clinical Psychiatry and a 2016 article in the journal Bipolar Disorders, researcher Jan Marie Kozicky and colleagues reported that in patients hospitalized with a first episode of mania, cognitive functioning and brain imaging abnormalities, respectively, returned to normal over the next year only if the patients experienced no further mood episodes. The message is clear: we must treat the first episode of mania comprehensively to avoid long-term deterioration, which occurs as a function of the number of episodes of mania or depression a patient experiences. However, this early multimodal approach is rarely taken in the US.
In schizophrenia, Dixon and colleagues noted that: “After the RAISE study reports were made available, Congress allocated additional funding to the community mental health …program, leading to growth in the number of…programs across the United States; they were expected to reach 48 states in 2018.”
The contrast between these efforts in schizophrenia and their virtual absence in bipolar disorder is incomprehensible and tragic. Studies in early schizophrenia have been funded for 25 years, while almost none have been funded in bipolar disorder, even in recent years. Community mental health programs for early schizophrenia will soon exist in 48 states; for patients with bipolar disorder there are no programs available in any state that I am aware of. The incidence of bipolar is about three times that of schizophrenia, and the long-term outcomes are often as devastating in bipolar disorder as in schizophrenia. There is a high incidence of drug abuse; social, educational and occupational deficits; and suicide in bipolar disorder. Early intervention with the many safe supplements, nutraceuticals, and well-tolerated drugs that are currently available to adult patients should be studied in young people with bipolar disorder, but such studies neither being funded nor conducted.
The reality is that childhood-onset bipolar disorder is poorly recognized and treated in the US, largely because of a paucity of treatment-related studies and knowledge about the best options for these young patients. If a reader of the BNN knows how to influence advocacy groups, leaders in the Substance Abuse Mental Health Services Administration (SAMHSA) and the National Institutes of Mental Health (NIMH), or influential politicians, it would be useful to take the initiative in bringing some of these deficits and disparities to their attention. Something must be done; ideas about how to do it are welcome. My own efforts to get funding for a childhood-onset bipolar research network in collaboration with such luminaries in the field as David Miklowitz (UCLA), Kiki D. Chang (Stanford University), Boris Birmaher (University of Pittsburg), Benjamin Goldstein (Stonybrook Research Institute), Eric Youngstrom (UNC, Chapel Hill), Soledad Romero (Hospital Clinic of Barcelona), and Josefina Castro Fornieles (University of Barcelona) have not been successful. We will keep trying, but the field needs to reach beyond the many investigators who are advocating for more treatment research to other people with more influence.
Curcumin Improves Memory and Depression
Recent studies suggest that curcumin, the micronutrient in turmeric that gives Indian curry its bright color, may reduce depression and improve memory.
A 2018 study published in the American Journal of Geriatric Psychiatry by Gary Small, director of geriatric psychiatry at UCLA’s Longevity Center, and colleagues found that a curcumin supplement improved mild, age-related memory loss in people without dementia.
Forty adults between the ages of 50 and 90 received either placebo or 90 mg of Theracumin, a bioavailable form of curcumin, twice daily for 18 months. The participants took cognitive tests at the beginning of the study and every 6 months during the study. Thirty of the participants also received positron emission tomography (PET) scans upon beginning and ending the study to evaluate the appearance of plaques and tangles in their brains.
Participants who received curcumin saw improvements in verbal and visual memory and attention over the course of the study compared to those who received placebo. The curcumin participants also saw mild improvements in mood, and less accumulation of amyloid plaques and tau tangles in the amygdala and hypothalamus, brain areas that play a role in memory and emotion. A few participants had mild gastrointestinal effects after taking Theracumin.
In India, where diets are high in curcumin, there is a lower incidence of Alzheimer’s than in the west, and older people also have better cognitive performance than in the west.
Curcumin has anti-inflammatory, antioxidant, and neuroprotective properties. Researchers speculate that curcumin may reduce brain inflammation, which has been implicated in both depression and Alzheimer’s disease.
A 2017 meta-analysis by Qin Xiang Ng and colleagues of 6 studies of curcumin including a total of 377 patients found that the substance has significant antidepressant effects compared to placebo. Half of the studies also reported improvements in anxiety. No adverse events were reported. Ng’s meta-analysis was published in the Journal of the American Medical Directors Association.
Cannabidiol May Help Treat Schizophrenia
 A 2017 article by researcher Philip McGuire and colleagues in the American Journal of Psychiatry reports that when added to antipsychotic medication, cannabidiol, a component of marijuana, improved positive symptoms of schizophrenia, such as hallucinations and delusions, more than did the addition of a placebo.
A 2017 article by researcher Philip McGuire and colleagues in the American Journal of Psychiatry reports that when added to antipsychotic medication, cannabidiol, a component of marijuana, improved positive symptoms of schizophrenia, such as hallucinations and delusions, more than did the addition of a placebo.
In the double-blind, parallel-group study, 43 participants received 1000 mg/day of cannabidiol in addition to their regular antipsychotic medication, while 45 participants received a placebo alongside their regular medication.
Side effects were minimal, and after six weeks those who received cannabidiol had decreased positive symptoms and were more likely to be considered improved and not severely unwell.
Editor’s Note: It is important to emphasize that cannabidiol is only a minor component of marijuana, which contains much more tetrahydrocannabinol (THC), which is psycho-mimetic, i.e. it can worsen psychosis. Pure cannabidiol is not readily available to the public.